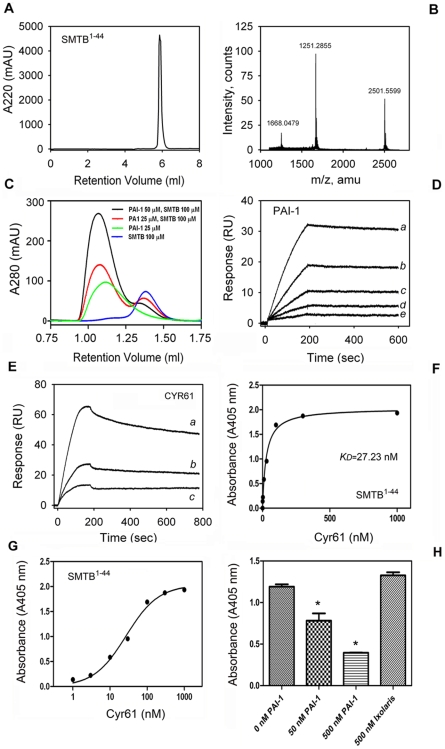
Figure 3. Cyr61 displays high-affinity binding to SMTB 1–-44 domain.
(A) SMTB 1–44 was chemically synthesized and purified by reverse-phase chromatography. (B) Mass spectrometry for the synthetic peptide shows the expected mass for SMTB 1–44. (C) Gel-filtration chromatography shows complex formation between PAI-1 and SMTB 1–44 was performed as in Materials and Methods. (D) SPR experiments. Sensorgrams shows PAI-1 (in nM: a, 1.8; b, 0.9; c, 0.45; d, 0.225; e, 0.11) binding to immobilized SMTB 1–44 domain. (E) Sensorgrams show Cyr61 (in nM: a, 12.5; b, 6.25; c, 3.1) binding to the SMTB 1–44 domain. Data were fitted using global two-state binding model. RU, resonance units. (F) Solid-phase binding assay. Cyr61 (0–1 µM) was incubated with immobilized SMTB 1–44 and binding estimated with anti-Cyr61 monoclonal antibody, followed by alkaline phosphatase-labeled anti-mouse secondary antibody and appropriate substrate as described in Materials and Methods. (G) Semi-log transformation of the results presented in (F). The (apparent) KD values for Cyr61/ SMTB 1–44 interactions were calculated by nonlinear regression analysis of the binding data according to the Langmuir isotherm equation. (H) Competition experiments. Cyr61 (30 nM) was incubated with 0, 50, and 500 nM PAI-1, or 500 nM ixolaris. Mixtures were added to SMTB 1–44-coated wells and incubated for 90 minutes. SMTB 1–44-bound Cyr61 was estimated as in (E). Experiments were performed in quadruplicate or quintuplicate (n = 3).