Abstract
Pulmonary hypertension (PH) is a severe, life-threatening disease for which there are no effective curative therapies. A diverse group of agents such as prostacyclins, endothelin antagonists, phosphodiesterase inhibitors, calcium channel blockers, diuretics, inotropic agents, and anticoagulants are used to treat PH; however, none of these agents have a marked effect upon survival. Among the new agents that show promise in the treatment of PH are Rho-kinase inhibitors and soluble guanylate cyclase stimulators. Although these new classes of agents have beneficial effects in experimental animal models and clinical studies, they are not selective in their actions on the pulmonary vascular bed. This manuscript reviews the actions of Rho-kinase inhibitors and soluble guanylate cyclase stimulators on the pulmonary vascular bed. It is our hypothesis that these new agents may be more effective than current therapies in the treatment of PH. Moreover, new methods in the delivery of these agents to the lung need to be developed so that their main effects will be exerted in the pulmonary vascular bed and their systemic effects can be minimized or avoided.
Keywords: RhoA/Rho-kinase pathway, smooth muscle, vascular, pulmonary hypertension, inhibitors/pharmacology, cardiovascular diseases, Fasudil, HA-1077, Y-27632, SB-772077-B, soluble guanylate cyclase, riociguat
Pulmonary arterial hypertension (PH) is a rare disorder that without treatment is progressive and often fatal within 3 years.1–4 The most common symptom at presentation is dyspnea with other complaints including fatigue, chest pain, syncope, leg edema, and palpitations.1–4 PH is also associated with other conditions or risk factors (e.g., collagen vascular diseases, portal hypertension, human immunodeficiency virus infection, drug use, toxins, dietary products, persistent fetal circulation, chronic thromboembolism, cancer, and diastolic heart failure,5–17 and the incidence of this disorder is increasing.
A national registry was begun in 1911 to collect medical data from 32 centers in patients diagnosed by uniform criteria as having PH [18]. One hundred eighty seven patients were entered into the registry with a mean age of 37 years (1–81 yrs) and with a female to male ratio of 1:1.7.18 Pulmonary function studies showed mild restriction (forced vital capacity 82% of predicted) with a reduced diffusing capacity for carbon monoxide, and hypoxemia with hypocapnia.18 The mean (± SD) right atrial pressure was 9.7 ± 6 mm Hg; mean pulmonary artery pressure (mPAP), 60 ± 18 mm Hg; cardiac index, 2.3 ± 0.9 L/min × m2; and pulmonary vascular resistance (PVR) index, 26 ± 14 mm Hg/L/min × m2 for this patient group.18 Raynaud’s phenomena was present in 10% of the patients and 95% of the patients were female.18 This National Institutes of Health-Primary PH registry represented a very important mechanism for characterizing the disease and the efficacy of therapeutic agents. In this registry, the median survival period was 2.8 years. 19
TREATMENT OF PULMONARY HYPERTENSION
The treatment of primary PH involves the use of a diverse group of drugs and lung transplantation. 20–35 Agents that are used to treat PH include oxygen, prostacyclin, and prostacyclin analogs, calcium channel blocking agents, phosphodiesterase (PDE5) inhibitors, endothelin antagonists, diuretics, digitalis, heparin, hydralazine, and angiotensin converting enzyme inhibitors; 25,36–40 and the development of a new class of Rho-kinase (ROCK) inhibitor such as fasudil has been used in the treatment of PH in small series of patients. 41–43 In previous times, alpha- and beta-adrenergic blockers and other vasodilating agents were used without favorable results. 44,45 To date the most effective treatment for primary PH is continuous intravenous administration of prostacyclin (Flolan™).46,47 The concept that prostacyclin, a natural product of cycloxygenase metabolism in the body, might have a beneficial effect in the treatment of PH was introduced when it was reported that a prostacyclin analog had unusual pulmonary vasodilator activity in the pulmonary vascular bed of the dog, and it was suggested that prostacyclin and analogs of prostacyclin might be used in the treatment of PH. 48 The pulmonary vasodilator actions of prostacyclin were characterized in the late ‘70s by Hyman and coworkers. 48–50 It is known that prostacyclin can improve exercise capacity and increase cardiac output. 46,51 Prostacyclin has been used in the treatment of PH since the late 70s, 52,53 and there are many series reporting the efficacious use of intravenous prostacyclin.54–59 Flolan™ (epoprostenol sodium) has been shown to improve exercise tolerance; however, this agent does not confer a survival benefit. 60 In these early studies, prostacyclin was given by continuous intravenous infusion requiring a pump and a long-term centrally-placed intravenous catheter. 57,58,61–64 There can be serious side effects such as hypotension, pain, and headache that can limit the use of this agent. 46,51 Prostacyclin and analogues can be given by other routes of administration, such as via inhaled65–70 and subcutaneous71–73 methods, but evidence is lacking that these routes are as effective as intravenous administration. 46,51 Interventions have been investigated such as adrenomedullin, 74–76 calcitonin gene-related peptide, 77,78 and vasoactive intestinal polypeptide,79 with the latter used in a small clinical study with positive effects.80 Stem-cell treatments have shown experimental benefit in small clinical studies,81–83 and it is possible that stem-cells engineered with therapeutic genes such as endothelial nitric oxide synthase (eNOS), adrenomedullin, calcitonin gene-related peptide, pituitary adenylate cyclase-activating polypeptide, or vasoactive intestinal polypeptide may be efficacious.77,82–87
ROCK inhibitors are a new class of agents which may be beneficial in the treatment of PH and a variety of other cardiovascular diseases, including angina, 88–94 heart failure, 95–97 cerebral vasospasm, 98–101 and systemic hypertension. 102–103 The chemical structures of currently studied ROCK inhibitors are shown in figure 1. Fasudil and Y-27632 are the first generation agents that have been widely studied, with fasudil showing efficacy in human studies in Japan and the United States. 42,43 SB-772077-B is a recently developed aminofurazan-based ROCK inhibitor with strong anti-inflammatory properties. 104
Figure 1.
Chemical structures of the Rho-kinase inhibitors which are currently available. Fausdil has been used in clinical studies in Japan and USA.
Vascular Smooth Muscle Contraction
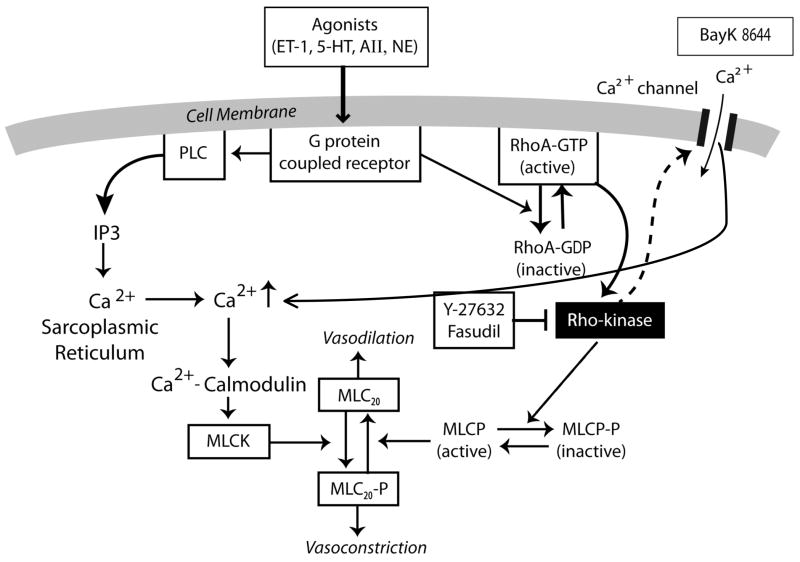
Vascular smooth muscle cell (VSMC) contraction is regulated by cytosolic Ca2+ levels ([Ca2+]i) as shown in figure 2.105–107 Elevation of [Ca2+]i is triggered by voltage-gated Ca2+ channels in the sarcolemma and by voltage-independent Ca2+ channels. 106 Voltage-independent Ca2+ channels or non-selective cation channels include receptor-operated channels, store-operated channels that are activated by the release of intracellular Ca2+ from the sarcoplasmic reticulum and by mechanosensitive, or stretch-activated channels, activated by membrane stretch. 106,107 With an elevation of [Ca2+]i, increases in the Ca2+-calmodulin complex occur with activation of myosin light chain kinase (MLCK). MLCK phosphorylates the 20-kDa regulatory MLC (MLC20) at either serine-19 or threonine-18 and results in activation of myosin ATPase that enhances cross-bridge cycling and induces VSM contraction (Fig. 2). However, with decreases in [Ca2+]i, a decrease in MLCK activity occurs, MLC20 is dephosphorylated by the action of MLC phosphatase, and VSM relaxation occurs (Fig. 2). Therefore, the reversible phosphorylation of MLC20 plays an essential role in the regulation of VSM contraction (Fig. 2). 105
Figure 2.
Diagram drawing showing interactions of receptor and store-operated calcium channels and of Rho-kinase in activation of myosin light chain (MLC20) in vascular smooth muscle (VSM). Phosphorylation of MLC20 and resultant VSM contraction are primarily determined by the balance between myosin light chain kinase (MLCK) that induces VSM contraction, and MLC phosphatase (MLCP) that induces VSMC relaxation. Increases in intracellular Ca2+ concentrations, Ca2+ entry (Bay K-8644, a Ca2+channel opener), and from receptor-operated calcium channels (G protein coupled receptor due to agonist interactions with ET-1, endothelin-1; 5-HT, serotonin; AII, angiotensin II; NE, norepinephrine; or TxA2, thromboxane A2) with subsequent Ca2+ release from the sarcoplasmic reticulum (SR), increase intracellular Ca2+-calmodulin complex that stimulates MLCK and induces VSM contraction. In the resting state, RhoA/GDP exists in the cytosol. However, with activation of certain trimeric G proteins, RhoA/GDP migrates and is changed to RhoA/GTP (now active) on the membrane of the VSM cell where it interacts with Rho-kinase to initiate increased phosphorylation of MLCP (active) to MLCP-P (inactive). MLCP opposes the action of MLCK. MLCP, myosin light chain phosphatase (active); MLCP-P, myosin light chain phosphatase (inactive); PLC, phospholipase C; IP3, inositol triphosphate. Modified from Nagaoka T, Fagan KA, Gebb SA, et al.: Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 2005;171:494–499.
Studies employing permeabilized smooth muscle cells demonstrated that a G-protein-coupled mechanism is also involved in “sensitization” of the contractile proteins to Ca2+.108 Since the discovery of this Ca2+-independent phase of vasoconstrictor tone, many studies have examined the intracellular signaling pathway that regulates Ca2+-sensitization, myosin activity, and VSM tone.105 Moreover, alterations in this Ca2+-independent signaling have subsequently been shown to play a major role in VSM dysfunction observed in a number of cardiovascular diseases including PH.109
RhoA and Rho-kinase pathway
RhoA and its downstream effector, ROCK, play an important role in the pathogenesis of PH. Knowledge about this pathway has been derived from clinical studies, in-vitro smooth muscle and isolated lung studies, and in chronic injury models such as chronic hypoxia- and monocrotaline- induced PH. 42,43,110–115 Chronic hypoxia-induced augmentation of RhoA- and ROCK- mediated pulmonary VSM Ca2+-sensitization were associated with elevated RhoA and ROCK activity and increased ROCK expression. 110 Pulmonary VSM sensitivity to NO is enhanced in rats by chronic hypoxia, as studies have shown that chronic hypoxia mediates a shift in NO signaling to promote pulmonary VSM Ca2+-sensitization through inhibition of the RhoA/ROCK pathway.110 Moreover, inhibition of ROCK abolishes the sustained phase of hypoxic vasoconstriction in rat pulmonary arteries and in perfused rat lungs. 111,112 However, in the intact chest rat, the ROCK inhibitors suppress all components of the hypoxic pulmonary vasconstrictor response. 116–120 In a recent study in monocrotaline-treated rats, the role of the ROCK pathway in the pathogenesis of fatal PH was investigated and results of the study indicated that the ROCK pathway was upregulated and that treatment with a ROCK inhibitor had a beneficial effect. 113 Similar results were observed in another experimental model of monocrotaline-induced PH in rats, and that ROCK activity was observed to be upregulated in pulmonary arteries but not in systemic vessels. 114 A recent study evaluated the role of ROCK activity in endothelial-denuded pulmonary arteries under elevated pressure conditions and observed that under chronic hypoxia conditions, ROCK inhibition produced a concentration-dependent reduction in but had no effect in control vessels. 115
In one clinical study, 8 female patients with idiopathic or collagen vascular disease PH underwent right heart catheterization and were treated with an IV infusion of fasudil, 1 mg/min over a 30-minute period. The infusion of the ROCK inhibitor produced significant decreases in PVR (~24%) and small but significant decreases in mPAP from 41±13 mm Hg to 38±15 mm Hg. 42 Cardiac index increased from 2.4±0.7 L/min/m2 to 2.8±0.8 L/min/m2. Systemic vascular resistance (SVR) and systolic systemic arterial pressure (SAP) were decreased, but the decrease in the latter was small. 42 In a second clinical study, 9 patients with PH underwent right heart catheterization and were administered IV fasudil, 30 mg over a 30-minute period. 43 In this study, a significant decrease in PVR (~17%) was also observed in this group, along with an observed increase in cardiac output, but without a significant change in SAP.43 These findings suggest that when ROCK is upregulated in the lung, a selective decrease in PAP could be observed.42,43
Y-27632
Although it is known that Y-27632 decreases PAP in rodents with monocrotaline-and chronic hypoxia- induced PH and that the response to acute hypoxia is reversed, 111,112,120–130 less is known about responses to this Rho-kinase inhibitor when tone is increased on an acute basis with vasoconstrictor agonists. Activation of the thromboxane receptor to induce VSM contraction has been suggested to occur through activation of ROCK. 131 Moreover, responses to Y-27632 have not been compared in the pulmonary and systemic vascular beds in an intact animal preparation. 113.122.126.132 In experiments in the intact chest rat, the administration of Y-27632 decreased PAP and SAP in a dose-related manner. 116 Inasmuch as cardiac output was not decreased and left ventricular end-diastolic pressure, as a measure of left atrial pressure, was unchanged, the decreases in pressure reflect decreases in PVR and SVR. The decreases in PAP were modest under baseline conditions, reflecting the low level of tone in the pulmonary vascular bed and were similar to responses to other vasodilator agents under baseline tone conditions.116 However, when baseline tone was increased with U-46619 infusion, the IV injections of Y-27632 produced larger dose-dependent decreases in PAP. These data are in agreement with the observation that the vasoconstrictor effect of thromboxane receptor activation is dependent upon ROCK activation. 133 Studies have examined the role of thromboxane-induced vascular resistance in an isolated lung preparation in the rat, and following ROCK inhibition with Y-27632, attenuated pulmonary vasoconstrictor responses to the thromboxane mimic, U-46619, were observed. 134 These studies are in agreement with other studies showing that Y-27632 and fasudil caused complete relaxation of U-46619-induced vasoconstriction in human internal mammary arteries, 135 whereas Y-27632 could only partially inhibit U-46619-induced contractions in bovine middle cerebral arteries. 136
Additionally, the effects of acute hypoxia on the pulmonary vascular bed and the response to Y-27632 during acute hypoxia were also investigated in the intact chest rat model. Ventilation with the 10% O2/90% N2 gas mixture increased PAP. 116 SAP and cardiac output were decreased and cardiac output returned back towards control values during the period of hypoxia. The administration of the potent ROCK inhibitor, Y-27632, reversed the pulmonary hypertensive response to acute ventilatory hypoxia. Moreover, prior administration of Y-27632 before ventilation with the hypoxic gas mixture prevented the increase in PAP in response to hypoxic gas ventilation.116
The decreases in PAP and SAP in response to Y-27632 and to fasudil were compared in U-46619-infused animals, and showed that the dose response curves for fasudil are approximately one half log unit to the right of the curves for Y-27632, indicating greater potency for Y-27632, and that both agents have similar efficacy and similar effects on the pulmonary and systemic vascular beds in the rat.116
The observation that Y-27632 promoted vasodilation under resting and under enhanced tone conditions provides support for the hypothesis that the Rho-kinase Ca2+ sensitization pathway is tonically active under physiologic conditions. The data indicate that the relative decreases in PAP and SAP under elevated tone conditions are similar, suggesting that ROCK-mediated Ca2+ sensitization plays an important role in both vascular beds and that the highly selective ROCK inhibitor, Y-27632, does not have selective pulmonary vasodilatory activity in the intact chest rat under physiologic conditions. 116 Finally, the observation that Y-27632 can promote vasodilation when tone is increased by diverse mechanisms, may in part explain the beneficial effects of ROCK inhibition in the treatment of cardiovascular diseases including PH.
These data are in contrast with other studies that have shown that the ROCK inhibitor, fasudil, has selective vasodilator activity in the rat, but without decreasing SAP.114 In this study of monocrotaline-induced PH in rats, decreases in the PAP were seen in response to fasudil without a decrease in SAP. 114 In another study in a hypoxic-induced PH model, the short-term inhalation of Y-27632 produced decreases in mPAP without reducing mSAP, suggesting selective pulmonary vasodilator activity, whereas in the same study, acute oral administration of Y-27632 produced sustained decreases in both mPAP and mSAP. 120 These data suggest that when delivered locally to the lung by aerosol, a selective pulmonary vasodilator response could be achieved. 120 However, in studies with hypoxic ventilation, decreases in both PAP and SAP were observed and there was no selective effect. 116 Studies with novel ROCK inhibitors will be needed in order to produce a selective vasodilator effect.
SB-772077
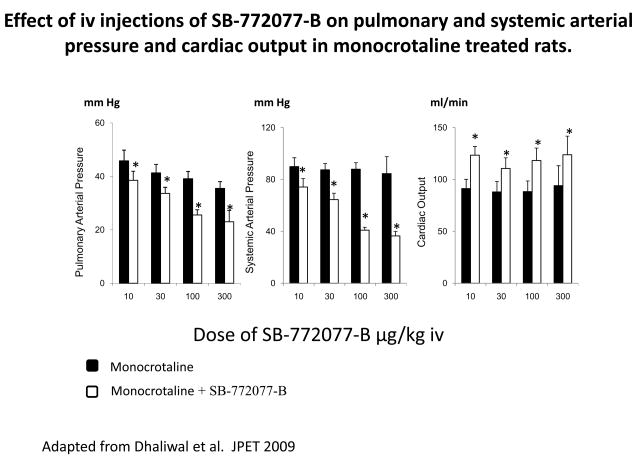
The novel ROCK inhibitor, SB-772077-B, has potent vasodilator activity in the pulmonary and systemic vascular beds in the rat and this ROCK inhibitor is shown to be more potent than the first generation ROCK inhibitors, Y-27632 or fasudil in U46619-infused animals. 117 This novel aminofurazan-based ROCK inhibitor had a beneficial effect in monocrotaline-inducedPH, and animals treated with SB-772077-Bfor 21 days had significantly lower PAPs compared with untreated control rats. 117 The effect of SB-772077-B in monocrotaline-treated rats with PH is shown in figure 3 where it can be seen that the ROCK inhibitor did not have a selective vasodilator effect in the pulmonary vascular bed. The comparison of responses to SB-772077-B in control and monocrotaline-treated animals can provide an estimate of the amount of reversible and fixed vasoconstrictor tone in the pulmonary vascular bed in animals with PH. 117
Figure 3.
Bar graphs comparing decreases in pulmonary and systemic arterial pressure and increases in cardiac output in response to IV injections of SB-772077-B in L-NAME treated animals. The IV injections of L-NAME in doses of 5 to 25 mg/kg produced a significant increase in pulmonary and systemic arterial pressures. n indicates number of experiments. * indicates significantly different from baseline. Modified from Dhaliwal JS, Badejo AM, Jr., Casey DB, et al.: Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin- 2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther 2009;330:334–341 [117].
The intravenous injection of 300 μg/kg SB-772077-B reversed the PH response to U46619 infusion, L-NAME (NG-nitro-L-arginine methyl ester) treatment, and ventilatory hypoxia, whereas in monocrotaline-treated animals, the IV injection of the ROCK inhibitor decreased PAP to 28 ± 2 mm Hg.117 These data suggest that approximately 60% of the vasoconstrictor tone is mediated by a SB-772077-B reversible mechanism in the monocrotaline-treated animal, and approximately 40% of the vasoconstrictor tone may represent fixed resistance, consistent with studies with fasudil in which right ventricular systolic pressure was measured. 122 The data in figure 4 show that SB-772077-B had potent pulmonary vasodilator activity when NO formation was inhibited and endothelial dysfunction was induced by L-NAME.
Figure 4.
Effect of IV injections of SB-772077-B on pulmonary and systemic arterial pressures and cardiac output in monocrotaline treated rats. The responses to SB-772077-B were measured on day 29 after treatment with the plant alkaloid in an IV dose of 60 mg/kg. Modified from Dhaliwal JS, Badejo AM, Jr., Casey DB, et al.: Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin- 2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther 2009;330:334–341 [117]
Recent studies have provided evidence that ROCK-mediated calcium sensitization plays an important role in the regulation of vasoconstrictor tone in the pulmonary vascular bed. 118,119 In addition to reversing PH responses to U46619, L-NAME, and ventilatory hypoxia, SB-772077-B decreased PAP in monocrotaline-treated rats with PH in which endothelial function is impaired. 137 These data are consistent with the hypothesis that the ROCK inhibitor can reverse PH independent of the mechanism used to promote vasoconstriction, including L-NAME-induced endothelial dysfunction (Fig. 4). The observation that increases in PAP in response to Bay K 8644 are attenuated by the ROCK inhibitor suggest that vasoconstrictor responses can be suppressed independent of the mechanism used to increase intracellular calcium levels.117 The intravenous injections of SB-772077-B in animals with monocrotaline-induced PH decreased PAPs and SAPs.117 The comparison of decreases in PAP and SAP in the PH animals indicates that SB-772077-B does not have selective pulmonary vasodilator activity. 117
Studies on the role of ROCK in the regulation of vasoconstrictor tone have been aided by the development of selective ROCK inhibitors. Results with the prototypical inhibitors, fasudil and Y-27632, have provided support for the concept that ROCK plays an important role in the regulation of baseline tone and vasoconstrictor responses in the pulmonary vascular bed. 118,119,126 SB-772077-B is a member of a novel class of aminofurazan-based inhibitors with anti-inflammatory activity and enhanced potency and selectivity for ROCK 1 and 2. 104 The intravenous injections of SB-772077-B decreased PAPs and SAPs and increased cardiac output. Inasmuch as left ventricular end-diastolic pressure is not changed, these data indicate thatSB-772077-B has significant vasodilator activity in the pulmonary and systemic vascular beds.117 The decreases in PAP were modest under baseline conditions when vasoconstrictor tone was low, but were enhanced when PVR was increased, and these data are similar to studies with PGI2 and other vasodilator agents in the pulmonary vascular bed. 49,118 Under elevated tone conditions, SB-772077-B was more potent than the prototypical inhibitors in decreasing PAP and SAP. Although dose-response curves for the novel aminofurazan inhibitor were 1 half-log unit and 1 log unit to the left of curves forY-27632 and fasudil, the three agents decreased PAP and SAP in a similar nonselective manner and had similar efficacy.
Rho-kinase Inhibitor Studies Summation
New information from recent studies is that administration of the ROCK inhibitor Y-27632 has vasodilator activity in the pulmonary and systemic vascular beds under normal physiologic conditions. Moreover, pulmonary vasodilator responses to Y-27632 are enhanced when baseline tone is increased by either the thromboxane A2 agonist, U-46619, or by the non-selective NOS inhibitor, L-NAME, and that the ROCK inhibitor prevents or reverses the response to acute hypoxia. 116 The results with the NOS inhibitor indicate that NO plays an important role in the maintenance of baseline tone and in modulating the response to hypoxia in the pulmonary vascular bed. Comparison of the relative decreases in PAP and SAP under elevated tone conditions indicates that the ROCK inhibitor has similar vasodilator activity in the pulmonary and systemic vascular beds. 116
The results of the present study show that chronic administration of SB-772077-B has a beneficial effect in the treatment of monocrotaline-induced PH. 117 In addition, this novel ROCK inhibitor had potent vasodilator activity in the pulmonary and systemic vascular beds and decreased PAP in monocrotaline-treated animals in a nonselective manner. The present data show that SB-772077-Battenuates pulmonary vasoconstrictor responses mediated by diverse mechanisms, including G-coupled receptor activation, enhanced calcium entry, hypoxia, and NOS inhibition. 117 Although SB-772077-Bwas more potent than the prototypical ROCK inhibitors fasudil and Y-27632, it was similar to these agents in that it does not have selective vasodilator effect in the pulmonary vascular bed. The experiments with SB-772077-B indicate that approximately 60% of the PH response to monocrotaline can be reversed by the ROCK inhibitor and that this represents the reversible component of PH in the monocrotaline-treated rat. 117 The present data suggest that chronic administration of SB-772077-B would be useful in the treatment of PH disorders, although this agent does not have selective pulmonary vasodilator activity. The results with the three different ROCK inhibitors show that this class of agents can promote vasodilation independent of the mechanism that induces vasoconstriction and will be useful in conditions in which endothelial function is impaired.
Calcium Entry Blocking Drugs
Hypoxia is the major cause of PH and right ventricular hypertrophy in chronic obstructive pulmonary disease, cystic fibrosis, kyphoscoliosis, high-altitude sickness, as well as seen in obesity-hypoventilation and sleep apnea syndromes. 138 PH results from chronic vasoconstriction from hypoxia resulting in muscular hypertrophy in the pulmonary arteries and arterioles. 138 However, these pathologic changes may regress if alveolar hypoxia can be corrected. 138 Although administration of oxygen therapy helps reverse hypoxic pulmonary vasoconstriction, patient noncompliance with oxygen therapy makes it difficult to achieve continual relief of alveolar hypoxia. Experimental findings indicate that hypoxic pulmonary vasoconstriction requires calcium influx and can be inhibited by certain slow-channel calcium blockers. 138 Calcium entry blocking drugs are the least intrusive drugs that could be used in the treatment of PH,139 and studies have shown significant unloading of the right ventricle without extensive remodeling of the pulmonary vascular bed. 140
Isradipine has potent vasodilator activity, but will produce systemic hypotension when systemically administered. 118,119 In a small clinical study with isradipine following 3 weeks of the treatment, a significant increase in the maximum power working capacity and performance were observed. Moreover, following 6 months of the treatment, there were positive trends in gas exchange: anaerobic threshold became higher and maximum oxygen consumption improved.141
Soluble guanylate cyclase
Direct stimulation of soluble guanylate cyclase (sGC) represents a promising therapeutic strategy for the treatment of PH. 142 Riociguat, a potent sGC stimulator, has an improved pharmacokinetic profile and has positive effects on pulmonary hemodynamics and exercise capacity in patients with PH. 142 Riociguat is currently being investigated in phase III clinical trials for the oral treatment of PH. The results of these studies show that PVR is decreased and cardiac function is improved in patients with moderate-to-severe PH. Riociguat offers great therapeutic potential as a treatment for patients with pulmonary vascular disorders, but does not have selective pulmonary vasodilator activity. 37,143
Nanoparticle Administration of Rho-kinase Inhibitors
Drug administration by inhalation is of interest to pulmonary medicine, and aerosol administration is an established method for the delivery of drugs to the lung for restrictive lung diseases such as asthma, chronic obstructive pulmonary disease, or bacterial infections of the lung. Beneficial features of inhalation therapy that are unique to the respiratory tract include the presence of a large surface area, short diffusion distances, and high blood perfusion. Recently, research on inhalation therapy with the use of nanoparticles (one billionth of a meter) has focused on the technology of aerosol science (solvent delivery vehicles) and these findings support our hypothesis that the inhalation delivery of a nanoparticle particle containing a ROCK inhibitor to the lung would be useful in the medical management of PH.
The development of drug delivery mechanisms to get drugs across cell membranes and into cells could be an advantage especially in pulmonary arterial smooth muscle cells where Ca2+-sensitization occurs. However, drug nanocarriers must be manufactured from biocompatible and biodegradable materials, and their quality, safety, and efficacy must be clearly demonstrated in pre-clinical animals studies measuring long-term outcomes and in carefully controlled clinical studies before widespread application in clinical medicine. Solid polymeric nanocarriers for insulin have been prepared from sodium cholate or soybean phosphatidylcholine and were observed to have a four-fold higher bioavailability when compared to subcutaneous application in a diabetic animal model. 144 Inhaled phospholipid nanocarriers have been shown to provide a controlled release of the encapsulated insulin.145 However, future experiments with long-term exposure in other animal models will need to show the therapeutic benefit of this strategy and minimal side effects of these nanocarrier molecules before bioavailability experiments in humans can be performed. It is our hypothesis that ROCK inhibition in the pulmonary circulation through the use of inhaled nanoparticle-encapsulated fasudil administered to the lung could promote selective pulmonary arterial vasodilation, reduce the development of pulmonary arterial muscularization and the development of right ventricular hypertrophy, three important findings in patients with PH. Moreover, the use of this novel technology could lead to more effective treatment modalities in the medical management of PH with a reduction in systemic effects.
Summary
These findings suggest that ROCK mediated calcium sensitization is a constitutively active process that plays a major role in the physiologic regulation of vasoconstrictor tone in the pulmonary and systemic vascular beds and in mediating pulmonary vasoconstrictor responses. The observation that ROCK inhibitors promote vasodilation when tone is increased by diverse mechanisms including NOS inhibition may, in part, explain the beneficial effects of ROCK inhibitors in cardiovascular diseases and other disorders including PH, in which endothelial dysfunction is present and vasoconstrictor tone is increased by diverse mechanisms which increase intracellular Ca2+ concentrations. Moreover, many studies suggest that the RhoA/ ROCK pathway has an important role in the early adaptive response of VSM under elevated tone conditions and support our hypothesis that inhibition of ROCK will promote vasodilation independent of the mechanism used to increase intracellular Ca2+ levels and induce vasoconstriction. It is our hypothesis that new agents like ROCK inhibitors and sGC stimulators such as riociguat which can activate sGC and promote pulmonary vasodilation when the heme iron moiety of sGC is oxidized or lost from the enzyme, will prove more effective than currently used agents in the treatment of PH. However, these new agents produce systemic hypotension and new routes and methods of administration, such as delivery of nanoparticles by inhalation that will produce a selective effect in the lung, will have to be developed.
Acknowledgments
NIH Grant HL 62000 and HL 77421
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005;143:282–292. doi: 10.7326/0003-4819-143-4-200508160-00009. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Widlitz A, Barst RJ. Pulmonary arterial hypertension in children. Eur Respir J. 2003;21:155–176. doi: 10.1183/09031936.03.00088302. [DOI] [PubMed] [Google Scholar]
- 4.Raiesdana A, Loscalzo J. Pulmonary arterial hypertension. Ann Med. 2006;38:95–110. doi: 10.1080/07853890600622143. [DOI] [PubMed] [Google Scholar]
- 5.Yang YY, Lin HC, Lee WC, et al. Portopulmonary hypertension: distinctive hemodynamic and clinical manifestations. J Gastroenterol. 2001;36:181–186. doi: 10.1007/s005350170126. [DOI] [PubMed] [Google Scholar]
- 6.Lai A, Frishman WH. Rho-kinase inhibition in the therapy of cardiovascular disease. Cardiol Rev. 2005;13:285–292. doi: 10.1097/01.crd.0000138079.91392.37. [DOI] [PubMed] [Google Scholar]
- 7.Palevsky HI, Gurughagavatula I. Pulmonary hypertension in collagen vascular disease. Compr Ther. 1999;25:133–143. doi: 10.1007/BF02889609. [DOI] [PubMed] [Google Scholar]
- 8.Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89:2722–2727. doi: 10.1161/01.cir.89.6.2722. [DOI] [PubMed] [Google Scholar]
- 9.Rich S, Levitsky S, Brundage BH. Pulmonary hypertension from chronic pulmonary thromboembolism. Ann Intern Med. 1988;108:425–434. doi: 10.7326/0003-4819-108-3-425. [DOI] [PubMed] [Google Scholar]
- 10.Rich S, Rubin L, Walker AM, et al. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest. 2000;117:870–874. doi: 10.1378/chest.117.3.870. [DOI] [PubMed] [Google Scholar]
- 11.Abenhaim L, Humbert M. Pulmonary hypertension related to drugs and toxins. Curr Opin Cardiol. 1999;14:437–441. doi: 10.1097/00001573-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Osba YK. Treatment of persistent pulmonary hypertension of the newborn: update. Arch Dis Child. 1991;66:74–77. doi: 10.1136/adc.66.1_spec_no.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis CS, Jr, Samuels AJ, Daines MC, et al. Chronic lung disease, polycythemia and congestive heart failure; cardiorespiratory, vascular and renal adjustments in cor pulmonale. Circulation. 1952;6:874–887. doi: 10.1161/01.cir.6.6.874. [DOI] [PubMed] [Google Scholar]
- 14.Ash R. Natural History of Ventricular Septal Defects in Childhood Lesions with Predominant Arteriovenous Shunts. J Pediatr. 1964;64:45–56. doi: 10.1016/s0022-3476(64)80315-2. [DOI] [PubMed] [Google Scholar]
- 15.Sackner MA, Akgun N, Kimbel P, et al. The Pathophysiology of Scleroderma Involving the Heart and Respiratory System. Ann Intern Med. 1964;60:611–630. doi: 10.7326/0003-4819-60-4-611. [DOI] [PubMed] [Google Scholar]
- 16.Brisbane JU, Howell DA, Bonkowsky HL. Pulmonary hypertension as a presentation of hepatocarcinoma. Report of a case and brief review of the literature. Am J Med. 1980;68:466–469. doi: 10.1016/0002-9343(80)90123-0. [DOI] [PubMed] [Google Scholar]
- 17.Abramson SV, Burke JF, Kelly JJ, Jr, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116:888–895. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 18.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 19.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 20.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001–2006. Heart. 2009;95:312–317. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 21.Toyoda Y, Thacker J, Santos R, et al. Long-term outcome of lung and heart-lung transplantation for idiopathic pulmonary arterial hypertension. Ann Thorac Surg. 2008;86:1116–1122. doi: 10.1016/j.athoracsur.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LJ. Pulmonary arterial hypertension. Proc Am Thorac Soc. 2006;3:111–115. doi: 10.1513/pats.200510-112JH. [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Branzi A. Pulmonary arterial hypertension: therapeutic algorithm. Ital Heart J. 2005;6:856–860. [PubMed] [Google Scholar]
- 24.Nauser TD, Stites SW. Pulmonary hypertension: new perspectives. Congest Heart Fail. 2003;9:155–162. doi: 10.1111/j.1527-5299.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 25.Sitbon O, Humbert M, Simonneau G. Primary pulmonary hypertension: Current therapy. Prog Cardiovasc Dis. 2002;45:115–128. doi: 10.1053/pcad.2002.128449. [DOI] [PubMed] [Google Scholar]
- 26.Franke UF, Wahlers T, Wittwer T, et al. Heart-lung transplantation is the method of choice in the treatment of patients with end-stage pulmonary hypertension. Transplant Proc. 2002;34:2181–2182. doi: 10.1016/s0041-1345(02)03195-0. [DOI] [PubMed] [Google Scholar]
- 27.Barst RJ. Recent advances in the treatment of pediatric pulmonary artery hypertension. Pediatr Clin North Am. 1999;46:331–345. doi: 10.1016/s0031-3955(05)70121-8. [DOI] [PubMed] [Google Scholar]
- 28.Stillwell PC, Mallory GB., Jr Pediatric lung transplantation. Clin Chest Med. 1997;18:405–414. doi: 10.1016/s0272-5231(05)70388-9. [DOI] [PubMed] [Google Scholar]
- 29.Kneussl MP, Lang IM, Brenot FP. Medical management of primary pulmonary hypertension. Eur Respir J. 1996;9:2401–2409. doi: 10.1183/09031936.96.09112401. [DOI] [PubMed] [Google Scholar]
- 30.Katz WE, Gasior TA, Quinlan JJ, et al. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J Am Coll Cardiol. 1996;27:384–391. doi: 10.1016/0735-1097(95)00502-1. [DOI] [PubMed] [Google Scholar]
- 31.Rich S. Medical treatment of primary pulmonary hypertension: a bridge to transplantation? Am J Cardiol. 1995;75:63A–66A. doi: 10.1016/s0002-9149(99)80385-3. [DOI] [PubMed] [Google Scholar]
- 32.Rubin LJ. Primary pulmonary hypertension. Practical therapeutic recommendations. Drugs. 1992;43:37–43. doi: 10.2165/00003495-199243010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Pasque MK, Trulock EP, Kaiser LR, et al. Single-lung transplantation for pulmonary hypertension. Three-month hemodynamic follow-up. Circulation. 1991;84:2275–2279. doi: 10.1161/01.cir.84.6.2275. [DOI] [PubMed] [Google Scholar]
- 34.Levine SM, Gibbons WJ, Bryan CL, et al. Single lung transplantation for primary pulmonary hypertension. Chest. 1990;98:1107–1115. doi: 10.1378/chest.98.5.1107. [DOI] [PubMed] [Google Scholar]
- 35.O’Meara N, Clarke R, Gearty G, et al. Primary pulmonary hypertension: treatment with heart-lung transplantation. Ir Med J. 1987;80:174–175. [PubMed] [Google Scholar]
- 36.Dranitsaris G, Mehta S. Oral therapies for the treatment of pulmonary arterial hypertension: a population-based cost-minimization analysis. Appl Health Econ Health Policy. 2009;7:43–59. doi: 10.1007/BF03256141. [DOI] [PubMed] [Google Scholar]
- 37.Mucke HA. Pulmonary arterial hypertension: on the way to a manageable disease. Curr Opin Investig Drugs. 2008;9:957–962. [PubMed] [Google Scholar]
- 38.LaRaia AV, Waxman AB. Pulmonary arterial hypertension: evaluation and management. South Med J. 2007;100:393–399. doi: 10.1097/SMJ.0b013e31802f2ff1. [DOI] [PubMed] [Google Scholar]
- 39.Benza RL, Park MH, Keogh A, et al. Management of pulmonary arterial hypertension with a focus on combination therapies. J Heart Lung Transplant. 2007;26:437–446. doi: 10.1016/j.healun.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 40.Kadowitz PJ, Hyman AL. Hydralazine and the treatment of primary pulmonary hypertension. N Engl J Med. 1982;306:1357–1359. doi: 10.1056/NEJM198206033062210. [DOI] [PubMed] [Google Scholar]
- 41.Li F, Xia W, Yuan S, et al. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol. 2009;30:363–366. doi: 10.1007/s00246-008-9315-z. [DOI] [PubMed] [Google Scholar]
- 42.Ishikura K, Yamada N, Ito M, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 43.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer M. Vasodilator therapy for primary pulmonary hypertension. Limitations and hazards. Ann Intern Med. 1985;103:258–270. doi: 10.7326/0003-4819-103-2-258. [DOI] [PubMed] [Google Scholar]
- 45.McGoon MD, Vlietstra RE. Vasodilator therapy for primary pulmonary hypertension. Mayo Clin Proc. 1984;59:672–677. doi: 10.1016/s0025-6196(12)62055-2. [DOI] [PubMed] [Google Scholar]
- 46.Paramothayan NS, Lasserson TJ, Wells AU, et al. Prostacyclin for pulmonary hypertension in adults. Cochrane Database Syst Rev. 2005:CD002994. doi: 10.1002/14651858.CD002994.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paramothayan NS, Lasserson TJ, Wells AU, et al. Prostacyclin for pulmonary hypertension. Cochrane Database Syst Rev. 2002:CD002994. doi: 10.1002/14651858.CD002994. [DOI] [PubMed] [Google Scholar]
- 48.Hyman AL, Chapnick BM, Kadowitz PJ, et al. Unusual pulmonary vasodilator activity of 13,14-dehydroprostacyclin methyl ester: comparison with endoperoxides and other prostanoids. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:5711–5715. doi: 10.1073/pnas.74.12.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyman AL, Kadowitz PJ. Pulmonary vasodilator activity of prostacyclin (PGI2) in the cat. Circulation Research. 1979;45:404–409. doi: 10.1161/01.res.45.3.404. [DOI] [PubMed] [Google Scholar]
- 50.Kadowitz PJ, Chapnick BM, Feigen LP, et al. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1978;45:408–413. doi: 10.1152/jappl.1978.45.3.408. [DOI] [PubMed] [Google Scholar]
- 51.Paramothayan NS, Lasserson TJ, Wells AU, et al. Prostacyclin for pulmonary hypertension. Cochrane Database Syst Rev. 2003:CD002994. doi: 10.1002/14651858.CD002994. [DOI] [PubMed] [Google Scholar]
- 52.Szczeklik J, Dubiel JS, Mysik M, et al. Effects of prostaglandin E1 on pulmonary circulation in patients with pulmonary hypertension. Br Heart J. 1978;40:1397–1401. doi: 10.1136/hrt.40.12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watkins WD, Peterson MB, Crone RK, et al. Prostacyclin and prostaglandin E1 for severe idiopathic pulmonary artery hypertension. Lancet. 1980;1:1083. doi: 10.1016/s0140-6736(80)91522-6. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin VV, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740–743. doi: 10.7326/0003-4819-130-9-199905040-00014. [DOI] [PubMed] [Google Scholar]
- 55.Conte JV, Gaine SP, Orens JB, et al. The influence of continuous intravenous prostacyclin therapy for primary pulmonary hypertension on the timing and outcome of transplantation. J Heart Lung Transplant. 1998;17:679–685. [PubMed] [Google Scholar]
- 56.Okano Y, Senju S, Tsutsui Y, et al. Long-term continuous intravenous infusion of prostacyclin for severe primary pulmonary hypertension. Intern Med. 1997;36:794–798. doi: 10.2169/internalmedicine.36.794. [DOI] [PubMed] [Google Scholar]
- 57.Barst RJ, Rubin LJ, McGoon MD, et al. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994;121:409–415. doi: 10.7326/0003-4819-121-6-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 58.Kramer MR, Fucks S, Simon Z, et al. Continuous therapy with intravenous prostacyclin for primary pulmonary hypertension: a bridge to heart-lung transplantation. Isr J Med Sci. 1993;29:613–616. [PubMed] [Google Scholar]
- 59.Scott JP, Higenbottam TW, Smyth RL, et al. Acute pulmonary hypertensive crisis in a patient with primary pulmonary hypertension treated by both epoprostenol (prostacyclin) and nitroprusside. Chest. 1991;99:1284–1285. doi: 10.1378/chest.99.5.1284. [DOI] [PubMed] [Google Scholar]
- 60.Macchia A, Marchioli R, Marfisi R, et al. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Archer-Chicko C. Continuous intravenous prostacyclin for advanced primary pulmonary hypertension. Dimens Crit Care Nurs. 2000;19:14–21. doi: 10.1097/00003465-200019020-00004. [DOI] [PubMed] [Google Scholar]
- 62.Dahlem P. Combination of inhaled nitric oxide and intravenous prostacyclin for successful treatment of severe pulmonary hypertension in a patient with ARDS. Intensive Care Med. 1999;25:1474–1475. doi: 10.1007/s001340051104. [DOI] [PubMed] [Google Scholar]
- 63.Barst RJ. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin. Heart. 1997;77:299–301. doi: 10.1136/hrt.77.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 65.Schenk P, Petkov V, Madl C, et al. Aerosolized iloprost therapy could not replace long-term IV epoprostenol (prostacyclin) administration in severe pulmonary hypertension. Chest. 2001;119:296–300. doi: 10.1378/chest.119.1.296. [DOI] [PubMed] [Google Scholar]
- 66.Della Rocca G, Coccia C, Costa MG, et al. Inhaled areosolized prostacyclin and pulmonary hypertension during anesthesia for lung transplantation. Transplant Proc. 2001;33:1634–1636. doi: 10.1016/s0041-1345(00)02623-3. [DOI] [PubMed] [Google Scholar]
- 67.Forrest IA, Small T, Corris PA. Effect of nebulized epoprostenol (prostacyclin) on exhaled nitric oxide in patients with pulmonary hypertension due to congenital heart disease and in normal controls. Clin Sci (Lond) 1999;97:99–102. [PubMed] [Google Scholar]
- 68.Barry PW. Unsuccessful treatment of pulmonary hypertension by inhaled nitric oxide and aerosolized prostacyclin. Anaesth Intensive Care. 1999;27:670–671. [PubMed] [Google Scholar]
- 69.Webb SA, Stott S, van Heerden PV. The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med. 1996;22:353–355. doi: 10.1007/BF01700458. [DOI] [PubMed] [Google Scholar]
- 70.Olschewski H, Walmrath D, Schermuly R, et al. Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med. 1996;124:820–824. doi: 10.7326/0003-4819-124-9-199605010-00006. [DOI] [PubMed] [Google Scholar]
- 71.Shapiro S, Hill NS. Transition from IV to subcutaneous prostacyclin: premature withdrawal? Chest. 2007;132:741–743. doi: 10.1378/chest.07-1992. [DOI] [PubMed] [Google Scholar]
- 72.van Albada ME, van Veghel R, Cromme-Dijkhuis AH, et al. Treprostinil in advanced experimental pulmonary hypertension: beneficial outcome without reversed pulmonary vascular remodeling. J Cardiovasc Pharmacol. 2006;48:249–254. doi: 10.1097/01.fjc.0000248229.87510.9b. [DOI] [PubMed] [Google Scholar]
- 73.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 74.Lu H, Chen S, Wang H, et al. Role of adrenomedullin in congenital heart disease associated with pulmonary hypertension. J Huazhong Univ Sci Technolog Med Sci. 2003;23:275–277. doi: 10.1007/BF02829512. [DOI] [PubMed] [Google Scholar]
- 75.Nagaya N, Kyotani S, Uematsu M, et al. Effects of adrenomedullin inhalation on hemodynamics and exercise capacity in patients with idiopathic pulmonary arterial hypertension. Circulation. 2004;109:351–356. doi: 10.1161/01.CIR.0000109493.05849.14. [DOI] [PubMed] [Google Scholar]
- 76.Vijay P. Adrenomedullin in the treatment of pulmonary hypertension. Heart. 2000;84:575–576. doi: 10.1136/heart.84.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Champion HC, Bivalacqua TJ, Toyoda K, et al. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation. 2000;101:923–930. doi: 10.1161/01.cir.101.8.923. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Q, Liu Z, Wang Z, et al. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg. 2007;84:544–552. doi: 10.1016/j.athoracsur.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 79.Haydar S, Sarti JF, Grisoni ER. Intravenous vasoactive intestinal polypeptide lowers pulmonary-to-systemic vascular resistance ratio in a neonatal piglet model of pulmonary arterial hypertension. J Pediatr Surg. 2007;42:758–764. doi: 10.1016/j.jpedsurg.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 80.Leuchte HH, Baezner C, Baumgartner RA, et al. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008;32:1289–1294. doi: 10.1183/09031936.00050008. [DOI] [PubMed] [Google Scholar]
- 81.Zeng C, Wang X, Hu X, et al. Autologous endothelial progenitor cells transplantation for the therapy of primary pulmonary hypertension. Med Hypotheses. 2007;68:1292–1295. doi: 10.1016/j.mehy.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 82.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 83.Tamm M, Gratwohl A, Tichelli A, et al. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Ann Rheum Dis. 1996;55:779–780. doi: 10.1136/ard.55.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao YD, Courtman DW, Deng Y, et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 85.Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi M, Nakamura T, Toba T, et al. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 87.Otto C, Hein L, Brede M, et al. Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation. 2004;110:3245–3251. doi: 10.1161/01.CIR.0000147235.53360.59. [DOI] [PubMed] [Google Scholar]
- 88.Otsuka T, Ibuki C, Suzuki T, et al. Administration of the Rho-kinase inhibitor, fasudil, following nitroglycerin additionally dilates the site of coronary spasm in patients with vasospastic angina. Coron Artery Dis. 2008;19:105–110. doi: 10.1097/MCA.0b013e3282f3420c. [DOI] [PubMed] [Google Scholar]
- 89.Otsuka T, Ibuki C, Suzuki T, et al. Vasodilatory effect of subsequent administration of fasudil, a rho-kinase inhibitor, surpasses that of nitroglycerin at the concentric coronary stenosis in patients with stable angina pectoris. Circ J. 2006;70:402–408. doi: 10.1253/circj.70.402. [DOI] [PubMed] [Google Scholar]
- 90.Inokuchi K, Ito A, Fukumoto Y, et al. Usefulness of fasudil, a Rho-kinase inhibitor, to treat intractable severe coronary spasm after coronary artery bypass surgery. J Cardiovasc Pharmacol. 2004;3:275–277. doi: 10.1097/01.fjc.0000134775.76636.3f. [DOI] [PubMed] [Google Scholar]
- 91.Hiroki J, Fukumoto Y, Shimokawa H, et al. Inhibition of Rho-kinase by fasudil preventing anginal attacks associated with spastic angina: a case report. J Cardiol. 2004;4:161–164. [PubMed] [Google Scholar]
- 92.Mohri M, Shimokawa H, Hirakawa Y, et al. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 93.Shimokawa H, Hiramori K, Iinuma H, et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 94.Masumoto A, Mohri M, Shimokawa H, et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 95.Chang J, Xie M, Shah VR, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kishi T, Hirooka Y, Masumoto A, et al. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 97.Schmitt M, Gunaruwan P, Payne N, et al. Effects of Exogenous and Endogenous Natriuretic Peptides on Forearm Vascular Function in Chronic Heart Failure. Arterioscler Thromb Vasc Biol. 2004;24:911–917. doi: 10.1161/01.ATV.zhq0504.7914. [DOI] [PubMed] [Google Scholar]
- 98.Hashiba Y, Tosaka M, Saito N, et al. Vasorelaxing effect of the Rho-kinase inhibitor, Y-27632, in isolated canine basilar arteries. Neurol Res. 2007;29:485–489. doi: 10.1179/016164107X164076. [DOI] [PubMed] [Google Scholar]
- 99.Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37:2174–2180. doi: 10.1161/01.STR.0000231647.41578.df. [DOI] [PubMed] [Google Scholar]
- 100.Tani E, Matsumoto T. Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol. 2004;2:13–21. doi: 10.2174/1570161043476492. [DOI] [PubMed] [Google Scholar]
- 101.Sato M, Tani E, Fujikawa H, et al. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- 102.Seasholtz TM, Wessel J, Rao F, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 103.Masumoto A, Hirooka Y, Shimokawa H, et al. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 104.Doe C, Bentley R, Behm DJ, et al. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther. 2007;320:89–98. doi: 10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- 105.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 106.Guibert C, Ducret T, Savineau JP. Voltage-independent calcium influx in smooth muscle. Prog Biophys Mol Biol. 2008;98:10–23. doi: 10.1016/j.pbiomolbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Salomonsson M, Sorensen CM, Arendshorst WJ, et al. Calcium handling in afferent arterioles. Acta Physiol Scand. 2004;181:421–429. doi: 10.1111/j.1365-201X.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- 108.Himpens B, Kitazawa T, Somlyo AP. Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Archiv Eur J Physiology. 1990;417:21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- 109.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1220–1229. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- 111.Robertson TP, Dipp M, Ward JP, et al. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z, Jin N, Ganguli S, et al. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- 113.Abe K, Shimokawa H, Morikawa K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 114.Jiang BH, Tawara S, Abe K, et al. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;49:85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- 115.Broughton BRS, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 116.Nossaman BD, Kadowitz PJ. The role of the RhoA/rho-kinase pathway in pulmonary hypertension. Curr Drug Discov Technol. 2009;6:59–71. doi: 10.2174/157016309787581057. [DOI] [PubMed] [Google Scholar]
- 117.Dhaliwal JS, Badejo AM, Jr, Casey DB, et al. Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther. 2009;330:334–341. doi: 10.1124/jpet.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Badejo AM, Jr, Dhaliwal JS, Casey DB, et al. Analysis of pulmonary vasodilator responses to the Rho-kinase inhibitor fasudil in the anesthetized rat. Am J Physiol Lung Cell Mol Physiol. 2008;295:L828–836. doi: 10.1152/ajplung.00042.2008. [DOI] [PubMed] [Google Scholar]
- 119.Dhaliwal JS, Casey DB, Greco AJ, et al. Rho kinase and Ca2+ entry mediate increased pulmonary and systemic vascular resistance in L-NAME-treated rats. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1306–1313. doi: 10.1152/ajplung.00189.2007. [DOI] [PubMed] [Google Scholar]
- 120.Nagaoka T, Fagan KA, Gebb SA, et al. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 121.Wang J, Weigand L, Foxson J, et al. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol. 2007;293:L674–685. doi: 10.1152/ajplung.00141.2007. [DOI] [PubMed] [Google Scholar]
- 122.Oka M, Homma N, Taraseviciene-Stewart L, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 123.Guilluy C, Rolli-Derkinderen M, Tharaux P-L, et al. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem. 2007;282:2918–2928. doi: 10.1074/jbc.M604195200. [DOI] [PubMed] [Google Scholar]
- 124.Gao Y, Portugal AD, Negash S, et al. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L678–684. doi: 10.1152/ajplung.00178.2006. [DOI] [PubMed] [Google Scholar]
- 125.Gao Y, Portugal AD, Negash S, et al. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L67–684. doi: 10.1152/ajplung.00178.2006. [DOI] [PubMed] [Google Scholar]
- 126.Nagaoka T, Morio Y, Casanova N, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 127.Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Lanner MC, Jin N, et al. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: role of Rho-kinase. Am J Respir Cell Mol Biol. 2003;29:465–471. doi: 10.1165/rcmb.2002-0157OC. [DOI] [PubMed] [Google Scholar]
- 129.McMurtry IF, Bauer NR, Fagan KA, et al. Hypoxia and Rho/Rho-kinase signaling. Lung development versus hypoxic pulmonary hypertension. Adv Exp Med Biol. 2003;543:127–137. [PubMed] [Google Scholar]
- 130.Khan I, Oriowo MA, Chandrasekhar B, et al. Attenuated noradrenaline-induced contraction of pulmonary arteries from rats treated with monocrotaline: role of rho kinase. J Vasc Res. 2005;42:433–440. doi: 10.1159/000087901. [DOI] [PubMed] [Google Scholar]
- 131.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 132.Abe K, Tawara S, Oi K, et al. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol. 2006;48:280–285. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- 133.Janssen LJ, Lu-Chao H, Netherton S. Excitation-contraction coupling in pulmonary vascular smooth muscle involves tyrosine kinase and Rho kinase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L666–674. doi: 10.1152/ajplung.2001.280.4.L666. [DOI] [PubMed] [Google Scholar]
- 134.Martin C, Goggel R, Ressmeyer AR, et al. Pressor responses to platelet-activating factor and thromboxane are mediated by Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L250–257. doi: 10.1152/ajplung.00420.2003. [DOI] [PubMed] [Google Scholar]
- 135.Batchelor TJ, Sadaba JR, Ishola A, et al. Rho-kinase inhibitors prevent agonist-induced vasospasm in human internal mammary artery. Br J Pharmacol. 2001;132:302–308. doi: 10.1038/sj.bjp.0703809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maeda Y, Hirano K, Nishimura J, et al. Rho-kinase inhibitor inhibits both myosin phosphorylation-dependent and -independent enhancement of myofilament Ca2+ sensitivity in the bovine middle cerebral artery. Br J Pharmacol. 2003;140:871–880. doi: 10.1038/sj.bjp.0705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 138.Kennedy TP, Michael JR, Summer W. Calcium channel blockers in hypoxic pulmonary hypertension. Am J Med. 1985;78:18–26. doi: 10.1016/0002-9343(85)90165-2. [DOI] [PubMed] [Google Scholar]
- 139.Kennedy TP, Michael JR, Huang CK, et al. Nifedipine inhibits hypoxic pulmonary vasoconstriction during rest and exercise in patients with chronic obstructive pulmonary disease. A controlled double-blind study. Am Rev Respir Dis. 1984;129:544–551. [PubMed] [Google Scholar]
- 140.Michael JR, Kennedy TP, Buescher P, et al. Nitrendipine attenuates the pulmonary vascular remodeling and right ventricular hypertrophy caused by intermittent hypoxia in rats. Am Rev Respir Dis. 1986;133:375–379. doi: 10.1164/arrd.1986.133.3.375. [DOI] [PubMed] [Google Scholar]
- 141.Chazova IE, Belenkov Iu N, Mareev V, et al. The effect of Lomir (isradipine) on the tolerance for physical loading and on the gas exchange indices of patients with primary pulmonary hypertension. Ter Arkh. 1994;66:54–57. [PubMed] [Google Scholar]
- 142.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33:785–792. doi: 10.1183/09031936.00039808. [DOI] [PubMed] [Google Scholar]
- 143.Anonymous Molecule of the month. Riociguat Drug News Perspect. 2009;22:167. doi: 10.1358/dnp.2009.22.3.1357787. [DOI] [PubMed] [Google Scholar]
- 144.Liu J, Gong T, Fu H, et al. Solid lipid nanoparticles for pulmonary delivery of insulin. Int J Pharm. 2008;356:333–344. doi: 10.1016/j.ijpharm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 145.Grenha A, Remunan-Lopez C, Carvalho EL, et al. Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins. Eur J Pharm Biopharm. 2008;69:83–93. doi: 10.1016/j.ejpb.2007.10.017. [DOI] [PubMed] [Google Scholar]