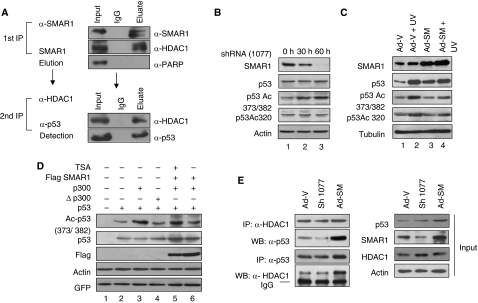
Figure 1.
SMAR1 interacts with and regulates p53 acetylation endogenously. (A) Double co-immunoprecipitation assay to check the in vivo association of SMAR1–HDAC1 complex with p53. One milligram of cell lysate from HCT116 p53+/+ cells was immunoprecipitated sequentially with SMAR1 and HDAC1 antibodies. The eluted fraction was probed with p53 antibody. (B) HCT116 p53+/+ cells were transfected with SMAR1 shRNA (sh1077). Western blotting shows endogenous acetylation status of p53 at lys 373/382 and total p53 level 30 and 60 h after transfection. (C) HCT116 p53+/+ cells transduced with GFP expressing control and SMAR1 adenoviruses and treated with UV (100 J/m2) for 24 h. The levels of p53 and p53 acetylation status in comparison with SMAR1 expression are shown. (D) In vitro deacetylation assay of p53 by SMAR1. HCT116 p53−/− cells were transfected with p53, p300 expression plasmids in different combinations and treated with TSA (200 nM, 16 h) as given in the figure. GFP expression plasmid was transfected to monitor transfection efficiency. (E) Reversible co-immunoprecipitation assay in HCT116 p53+/+ cells showing differential association of p53 with HDAC1 in SMAR1 knockdown and overexpressed cells (left panel). Input controls are shown in right panel.