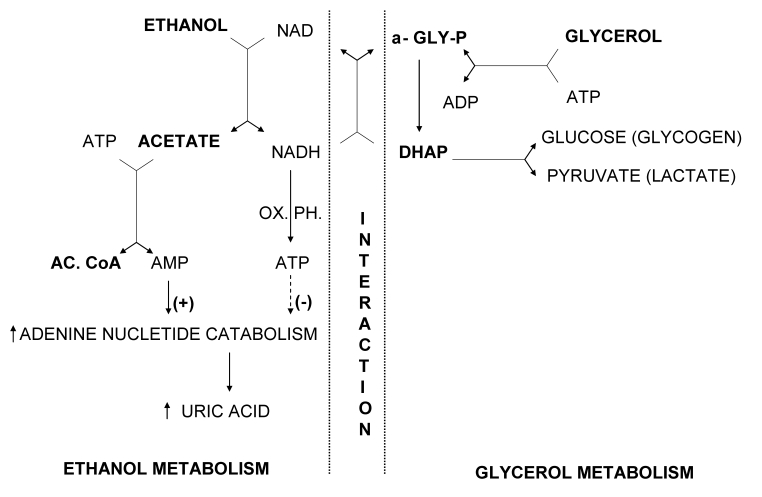
Figure 1.
Proposed biochemical mechanism of increased uric acid production induced by glycerol and ethanol (modified and updated after Lundquist et al (38). AMP – adenosine monophosphate; ADP – adenosine diphosphate; ATP – adenosine triphosphate; NAD – nicotinamide adenine dinucleotide; NADH – nicotinamide adenine dinucleotide (reduced form); AC. CoA – acetyl-coenzyme A; α-GLY-P – α-glycerophosphate; DHAP – dihydroxyacetone phosphate, OX. PH – oxidative phosphorylation. Ethanol is first oxidized to acetaldehyde, which is further oxidized to acetate. During these two oxidation steps, a parallel reduction of two molecules of NAD to NADH occurs. This generation of reducing equivalents in the liver is associated with ATP synthesis. Acetate is metabolized to acetyl-CoA via acetyl-AMP. During conversion of acetate to acetyl-AMP to acetyl-CoA, ATP is dephosphorylated to AMP; thus 2 mol of high-energy phosphate are consumed for each mol of ethanol metabolized. Although most of the AMP formed is resynthesized to ATP, a small amount of AMP may enter the pathway of adenine nucleotide degradation leading to uric acid production. ATP formed during ethanol oxidation to acetate is inhibitory to the adenine nucleotide degradation. The first step in glycerol metabolism is phosphorylation to α-glycerophosphate associated with dephosphorylation of ATP to ADP. The produced α-glycerophosphate is then oxidized to dihydroxyacetone phosphate (DHAP), which can be directed to production of glycogen (glucose) or pyruvate (lactate). In the presence of sufficient amounts of NADH, largely produced during ethanol metabolism, dihydroxyacetone phosphate can be reduced back to α-glycerophosphate. The net result of redirecting NADH to the production of α-glycerophosphate instead of ATP would be increased adenine nucleotide degradation and production of uric acid.