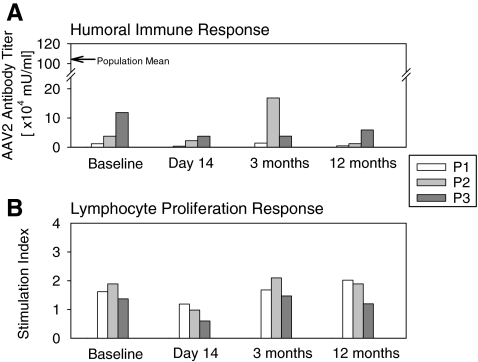
FIG. 1.
Immunological assays before surgery (baseline) and at 14-, 90-, and 365-day time points after retinal gene therapy. (A) Humoral immune response to AAV serotype 2 (AAV2) assayed by circulating serum antibody titers against AAV2 capsid in P1, P2, and P3 before and after surgery. Arrow along the vertical axis indicates mean levels from a normal reference population (n = 79) (Hauswirth et al., 2008). (B) Antigen-specific lymphocyte proliferation response (ASR) assayed in peripheral blood lymphocytes incubated in the presence versus in the absence of AAV2 capsid antigen. The stimulation index (the ratio of [3H]thymidine uptake in the presence of antigen to the uptake in its absence) in each subject after surgery is unchanged from baseline values.