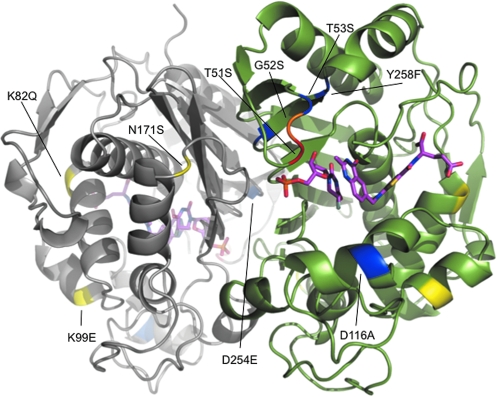
FIG. 2.
Amino acid substitutions in the eight mutants tested for 5-FUdR resistance in human cells are highlighted in the 1.9-Å crystal structure of dimeric human TS complexed with dUMP and the antifolate drug Raltitrexed. The closed conformation is shown. Residues 1–25 are disordered and do not appear in the model. The individual monomers are colored gray or green; for convenience, amino acid substitutions are indicated in only one of the monomers, although all substitutions are present in both. Comparison of LD50 values among mutants (Fig. 1b) permitted the following differentiation of resistance-contributory versus provisionally neutral amino acid substitutions: Substitutions that either alone or together conferred enhanced 5-FUdR resistance (T51S, G52S) are colored red and orange. Substitutions present in mutants that conferred improved 5-FUdR resistance, but appear to be autonomous of this selected property (K82Q, K99E, N171S), are shown in yellow. Substitutions that alone or together did not confer greater 5-FUdR resistance than wild-type TS (D254N, T53S, Y258S, D116A, Y258F) are shown in blue.