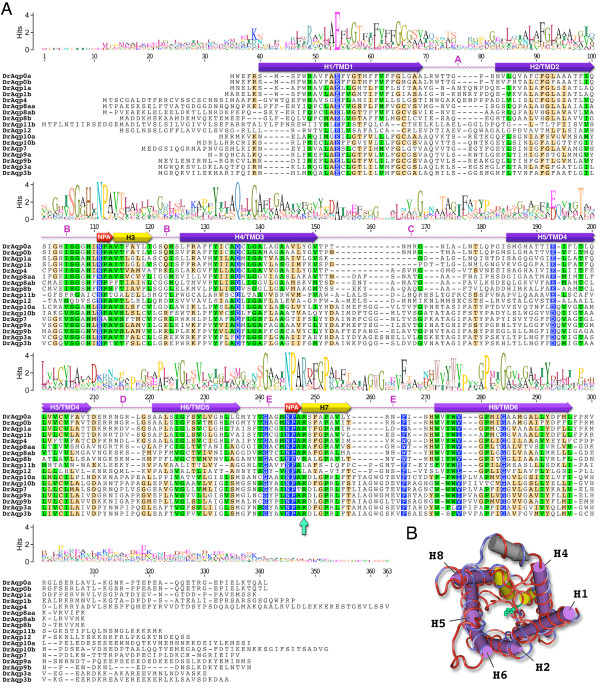
Figure 1.
Amino acid sequence alignment of zebrafish aquaporins. (A) The consensus sequence logo is scaled according to amino acid conservation. Highest residue similarity (blue: 100%, green: 80-100% or sand; 60-80%) is found within the α-helical regions (H1-8). The transmembrane domains (TMD1-6) are annotated for DrAqp0a based upon a molecular sequence wrap to the crystallographically resolved structure of Bos taurus AQP0 (B). The structure wrap consists of the complete peptides (263 amino acids) with a gapless identity/similarity of 70.3/85.9%. The render shows identical residues in red, non-identical in blue. The hemi-helices H3 and H7 (yellow) on loops B and E, respectively, fold such that the opposing NPA motifs (pink in the alignment) interact to present the arginine constriction (DrAqp0a R187 green ball and stick, and arrow in alignment). The C-terminal domain is shown with a grey α-helix.