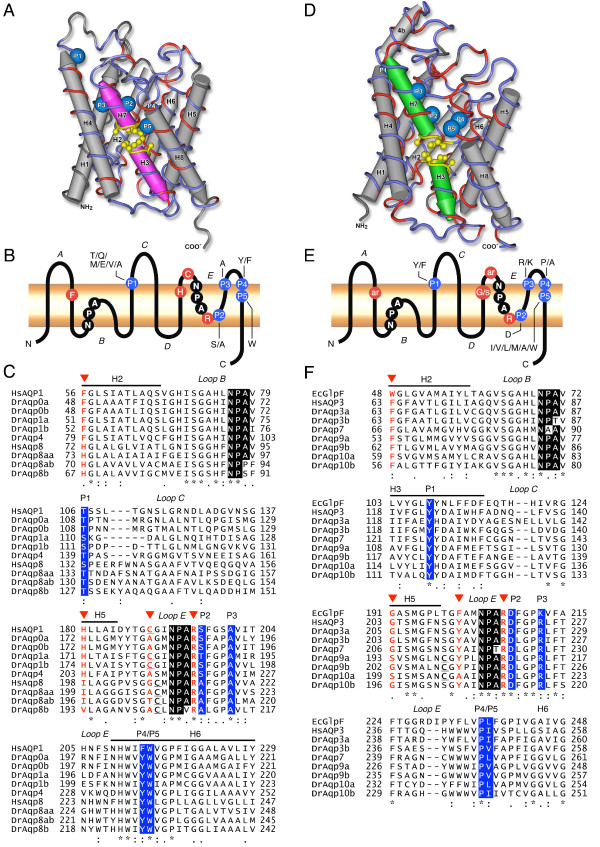
Figure 6.
Structural features of zebrafish aquaporins. (A-C) Water-selective aquaporins and aquaglyceroporins (Glps; D-F). (A and D) Three-dimensional reconstruction of DrAqp4 wrapped to the crystallographically resolved structure of Escherichia coli AqpZ (1RC2 chain B), and DrAqp3a wrapped to the crystallographically resolved structure of E. coli GlpF (1LDI chain A). Molecules are mirror tube-worm renders rotated to show identical (red) and non-identical (blue) residues and the annotated features including the blue space-filled conserved sites (P1-P5) and the opposing yellow ball and stick Asn-Pro-Ala (NPA) motifs between hemi-helices H3 and H7. Despite low primary identity/similarity (21.1/36.6% for DrAqp4; 34.1/53.0% for DrAqp3a) the secondary and tertiary structures appear conserved. (B and E) Schematic diagram of aquaporin monomers showing the 6 transmembrane helices (H), the two NPA motifs, the amino acids forming the aromatic/arginine (ar/R) constriction, and the five residues (P1-P5) conserved in water-selective (B) and Glps (E). In each position the conserved residues are indicated. (C) Amino acid sequence alignment of human AQP1 and AQP8 (HsAQP1 and HsAQP8), mouse AQP8 (MmAQP8), DrAqp0a, -0b, -1a, -1b, -4, and zebrafish AQP8-related sequences (DrAqp8aa, -8ab and -8b). The arrowheads point to the positions of the ar/R constriction. The P1-P5 conserved amino acids are shaded in blue. The asterisks indicate identical residues, whereas conserved amino acid substitutions and substitutions with similar amino acids are indicated by a double or single dot, respectively. The potential mercury-sensitive Cys site before the second NPA motif is underlined. (F) Amino acid sequence alignment of EcGlpF, human AQP3 (HsAQP3) and zebrafish Glps. Symbols and notes as in C.