Table 2.
Cyclopropanations of Chiral α-Substituted Allenamides.
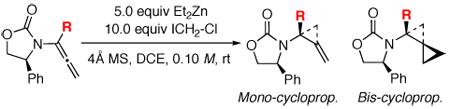 | ||||
|---|---|---|---|---|
| entry | allenamide | R = | time [h] | yield [%] [ratios]a |
| 1 | 32 | Me | 3 | 36-M: 38 [3.5:1] 36-B: 26 [3.5:1] |
| overall dr = 3.5:1b | ||||
| 2 | 32 | Me | 16 | 36-M: 19c [10.0:1] 36-B: 36 [3.0:1] |
| overall dr = 4.1:1b | ||||
| 3 | 33 | n-Bu | 3 | 37-M: 37d [5.0:1] 37-B: 18 [≥20:1] |
| overall dr = 7.9:1b | ||||
| 4 | 33 | n-Bu | 16 | 37-M: 30d [6.0:1] 37-B: 22 [≥20:1] |
| overall dr = 11.1:1b | ||||
| 5 | 34 | Bn | 16 | 38-M: 32 [7.0:1] 38-B: 20 [≥20:1] |
| overall dr = 12.0:1b | ||||
| 6 | 35 | CH2C6H4[o-Ph] | 3 | 39-M: 36 [5.0:1] 39-B: 23 [≥20:1] |
| overall dr = 8.8:1b | ||||
Isolated yields. Dr ratios are in the bracket with the respective major diastereomer being shown in the scheme and all ratios were assigned using crude 1H NMR.
Overall dr ratios represent the combined dr for the first cyclopropanation.
NMR yield.
See reference 42.
