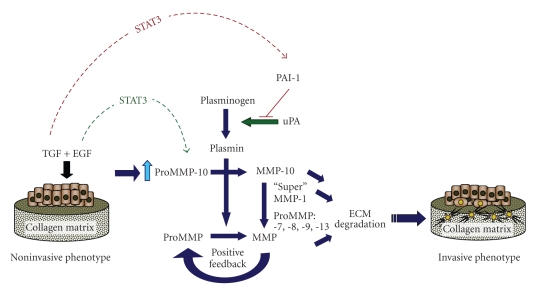
Figure 5.
Proposed mechanistic context for TGF-β1+EGF-enhanced plasmin-dependent collagen matrix remodeling and its contribution to development of an invasive phenotype. Dual growth factor-stimulated HaCaT II-4 keratinocytes cultured on a three-dimensional collagen gel upregulate critical stromal remodeling factors that both disrupt and preserve matrix integrity. In the presence of active plasmin, increased MMP-10 promotes MMP activation and creates a proteolytic axis that accelerates collagen degradation through “superactivation” of MMP-1. STAT3 phosphorylation may serve as a temporal switch in this process, through its ability to both promote EGF-stimulated proMMP-10 expression and antagonize induction of TGF-β1 target genes (i.e., PAI-1, fibronectin) [59]. The synergistic upregulation of PAI-1 in response to TGF-β1+EGF may subsequently shift this proteolytic balance, enabling PAI-1 to “titrate” the extent and locale of collagen matrix remodeling to facilitate tumor cell stromal invasion. Indeed, PAI-1 induction is an early event in this phenotypic transition and required for the motile response since PAI-1 knockdown (with siRNA constructs) effectively inhibited TGF-β1+EGF-initiated colony scattering [60].