TABLE 1.
Hydroboration/Protodeboronation of Alkynyl Pinacolboronates Bearing Various Functional Groups Pinacolboronates Bearing Various Functional Groupsa
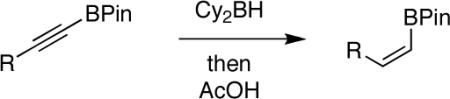
| entry | R | product | % isolated yield |
|---|---|---|---|
| 1 | 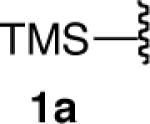 |
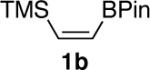 |
86 |
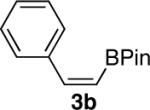
| 2 | 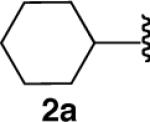 |
 |
84 |
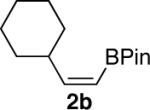
| 3 |  |
 |
83 |
| 4 | 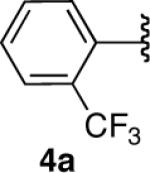 |
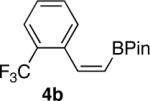 |
78 |
| 5 | 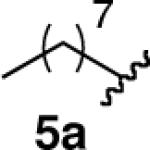 |
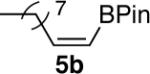 |
77 |
| 6 | 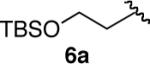 |
77 | |
| 7 | 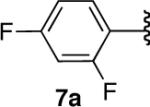 |
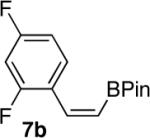 |
71 |
| 8 | 66b | ||
| 9 | 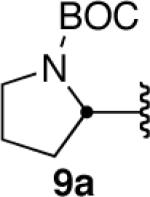 |
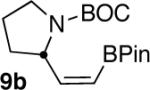 |
57c |
| 10 | 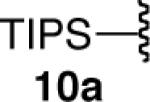 |
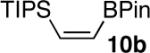 |
93d |
| 11 | 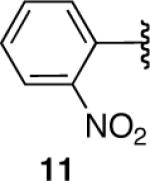 |
-e | |
| 12 | 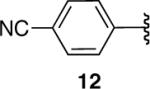 |
-e | |
| 13 | 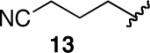 |
-e |
All reactions were carried out on 2 mmol scale in Et2O (4 mL) using 1:1 starting material/ borane. AcOH (2.2 equiv) was added at 0 °C, then ethanolamine at 0 °C and warmed to rt.
The product was isolated as a 90:10 mixture of cisltrans isomers.
The alkynyl pinacolboronate was not stable, necessitating that it be carried through without characterization.
The product was isolated with 10% of protodeboronation byproducts, which were inseparable by chromatrography or distillation.
Decomposition
