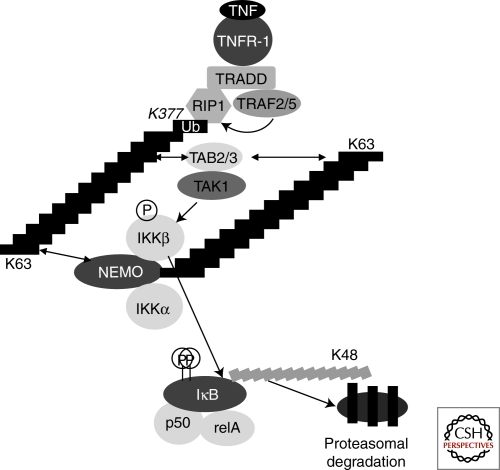
Figure 3.
The NF-κB response to TNF. TNF induces trimerization of the TNF receptor, leading to the recruitment of TRADD, the E3 ubiquitin-ligase TRAF2 (and/or TRAF5), and the kinase RIP1 (other recruited molecules have been omitted for simplicity). K63-linked polyubiquitination of RIP1 on Lys 377, possibly mediated by TRAF2/5 (curved arrow), leads to the recruitment of the TAK1/TAB1/TAB2 complex through the ubiquitin-binding zinc finger of TAB2 (TAB2 can be replaced by TAB3, and TAB1 has been omitted for clarity). Through an unknown mechanism, this leads to the activation of the TAK1 kinase. The IKK complex is also recruited to these K63 polyubiquitin chains through the ubiquitin-binding domain of NEMO, allowing TAK1 to phosphorylate and activate the IKKs. K63-linked polyubiquitination of NEMO has been observed, but its actual role is currently unclear. One possibility is that it allows recruitment of the TAK1 complex in close proximity to the IKK kinase subunits, allowing their activation by TAK1; alternatively it might allow NEMO oligomerization through cross-recognition by its own ubiquitin binding domain. Whatever the exact mechanism of activation of the IKKs, they eventually phosphorylate the IκBα inhibitory subunit of NF-κB. IκBα is then polyubiquitinated through Lys48-linked polyubiquitin chains by the β-TrCP E3 ubiquitin ligase, leading to its degradation by the proteasome and to nuclear translocation of free NF-κB dimers, ultimately ending in activation of NF-κB target genes.