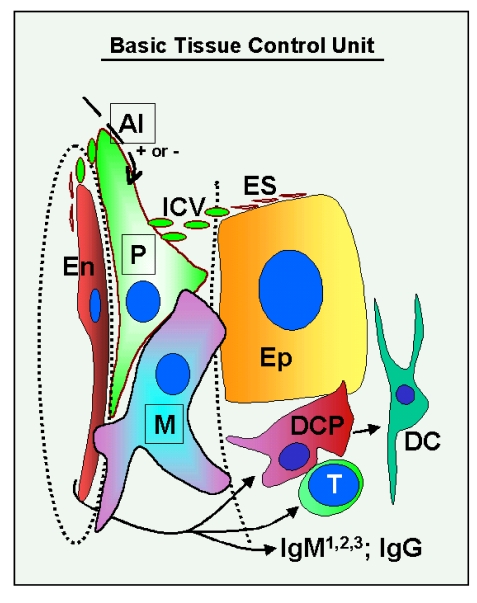
Figure 1. Schematic drawing of the basic "tissue control unit," which consists of monocyte-derived cells (marked M in the figure), vascular pericytes (P), and autonomic innervation (AI, dashed arrow), and the involvement of other components of the tissue control system (solid arrows).
Monocyte-derived cells physically interact with adjacent epithelial (Ep) and endothelial cells (En) through the basement membranes (dotted lines), and influence pericytes, which secrete intercellular vesicles (ICV). These vesicles collapse into the so-called empty spikes (ES) releasing their content (growth factor/cytokine) after reaching target cells. The activity of pericytes is stimulated or inhibited by autonomic innervation (+ or -) which controls quantitative aspects of tissues. Interaction of MDC with endothelial cells may stimulate homing of T lymphocytes (T) and monocyte-derived dendritic cell precursors (DCP; also known as veiled cells) differentiating into mature dendritic cells (DC). The dendritic cell precursors and T cells interact themselves and stimulate advanced differrentiation of epithelial cells. IgMs regulate early (IgM1), mid (IgM2), and late differentiation (apoptosis) of epithelial cells (IgM3), and IgG associates with aged cells (see Figure 2 and 3). The monocyte-derived cell system (including intraepithelial DCP and mature DC) is postulated to play a dominant role in the regulation of qualitative aspects of tissue-specific cells, including expression of ligands for intraepithelial T cells and regulating autoantibody action. Monocyte-derived cells also carry "stop effect" information (Figure 10B), presumptively encoded at the termination of immune adaptation (Figure 10A), which determines the highest state of epithelial cell differentiation allowed for a particular tissue. For details see Ref. [3,4,33]. Reprinted from Ref. [4], © Antonin Bukovsky.