Abstract
The importance of cytoplasmic motifs in differentially regulating SR-A function was demonstrated by deleting the first 49 cytoplasmic aa (SR-AΔ1–49), which abolished SR-A-mediated ligand internalization without reducing cell adhesion. To identify additional cytoplasmic motifs within the first 49 aa that regulate SR-A function, the acidic residues in a conserved motif (EDAD) were changed to their amide derivatives (SR-AQNAN). The function and regulation of SR-AQNAN were compared with that of SR-AΔ1–49 and SR-A in transfected HEK-293 cells. Blocking PI3K activation inhibited SR-A, but not SR-AΔ1–49- or SR-AQNAN-mediated cell adhesion. Although deleting (SR-AΔ1–49) or mutating (SR-AQNAN) the EDAD motif abolished the PI3K sensitivity of SR-A-mediated cell adhesion, these mutations did not affect ligand internalization or PI3K activation during cell adhesion. To define the mechanism by which PI3K regulates SR-A-mediated cell adhesion, the cellular localization of wild-type and mutant SR-A was examined. PI3K inhibition reduced surface localization of SR-A but not of SR-AΔ1–49 or SR-AQNAN. The regulation of SR-A surface localization by PI3K was confirmed in peritoneal macrophages, which endogenously express SR-A. Together, these results suggest a pathway in which SR-A binding to an immobilized ligand activates PI3K to recruit more receptor to the plasma membrane and enhances cell adhesion.
Keywords: macrophage, signal transduction, inflammation, structure-function
Introduction
SR-A are trimeric transmembrane glycoproteins that are expressed primarily by macrophages. SR-A binds a variety of ligands, including modified lipoproteins, bacterial products, and extracellular matrix proteins, and mediates ligand internalization and cell adhesion (reviewed in refs. [1, 2]). Because of its ability to bind diverse ligands and perform multiple functions, SR-A has the potential to be involved in many physiological and pathologic processes such as host defense, diabetes, and atherosclerosis. To date, most studies have focused on identifying SR-A ligands and the consequence of SR-A deficiency. Relatively few studies have assessed the relationship between SR-A structure and function or the molecular determinants that permit SR-A to mediate ligand internalization and adhesion.
Multiple adhesion substrates for SR-A, including glycated and denatured collagens, and proteoglycans present at sites of inflammation have been identified [3,4,5]. Several studies suggest that SR-A may be important for macrophage recruitment, adhesion, and retention at sites of tissue damage. For example, relative to macrophages isolated from wild-type mice, SR-A-deficient macrophages exhibit reduced spreading on tissue-culture plates [6], whereas peritoneal macrophages isolated from transgenic mice with macrophage-specific overexpression of SR-A showed enhanced spreading [7]. Moreover, SR-A-overexpressing transgenic mice displayed enhanced granuloma formation characterized by increased numbers of macrophages at the site of a s.c. injection of the SR-A ligand carrageenan [7]. Additional studies indicate that relative to naïve peritoneal macrophages, thioglycollate-elicited peritoneal macrophages displayed a significant increase in SR-A-mediated adhesion [8]. Thus, SR-A-mediated adhesion may contribute to macrophage accumulation at sites of inflammation or tissue damage.
Several studies have indicated that the N-terminal cytoplasmic tail regulates SR-A function. The cytoplasmic tail of human, bovine, and rabbit SR-A consists of 50 aa, whereas the murine cytoplasmic tail has 55 aa. The cytoplasmic tail of SR-A lacks a defined internalization motif; however, deletion or mutation of a conserved VKFD motif, corresponding to aa 26–29 in the murine sequence, decreased surface localization and ligand uptake, indicating that this motif is involved in regulating SR-A internalization [9]. Other studies have suggested that SR-A localization on the cell surface and its ability to internalize ligand are regulated by the activation of heterotrimeric G protein-regulated signaling pathways, phosphorylation of specific cytoplasmic serines, and interaction with certain cytoplasmic proteins [10,11,12]. However, these studies did not examine the potential role of specific cytoplasmic motifs in regulating SR-A-mediated cell adhesion.
Similar to integrin-mediated adhesion, SR-A mediates cell adhesion via the activation of several intracellular signaling molecules [13,14,15]. Activation of these signaling pathways results in formation of focal adhesions and cytoskeletal changes that promote cell adhesion. It was shown previously that the six membrane-proximal aa (SRAΔ1–49) were required for SR-A-mediated cell surface localization and cell adhesion but were not sufficient for receptor internalization [16]. The potential roles of additional cytoplasmic motifs in modulating SR-A-mediated cell adhesion have not been examined.
Cell adhesion is a complex process that involves the initial attachment of cells to substrate and the subsequent induction of a spread morphology that is characterized by an increase in surface area and organization of the actin cytoskeleton [14, 17, 18]. We showed previously that PI3K is activated during SR-A-mediated macrophage adhesion and that this activation is required for inducing a spread cell morphology and actin polymerization [14]. We have also shown that SR-A-mediated cell adhesion requires the 6 membrane-proximal aa of the receptor [16]. However, the importance of these 6 aa or of other cytoplasmic motifs in coupling SR-A to specific regulatory pathways has not been defined. Therefore, we examined the function and regulation of SR-A receptor constructs with an altered cytoplasmic sequence. Our results demonstrate that SR-A-mediated cell adhesion and surface localization, but not ligand internalization, are regulated by PI3K activation. Moreover, this PI3K sensitivity specifically requires an acidic aa-rich motif in the cytoplasmic tail of the receptor.
MATERIALS AND METHODS
Cell isolation and culture
MPM were harvested from NIH-Swiss mice (Jackson Laboratory, Bar Harbor, ME, USA) via peritoneal lavage with ice-cold sterile saline and cultured in DMEM (Gibco-BRL, Grand Island, NY, USA), supplemented with L-glutamine, heat-inactivated FBS (10% vol/vol), and penicillin/streptomycin [10]. Animal care and use for all procedures were done according to protocols reviewed and approved by the Institutional Animal Care and Use Committee at the University of Kentucky (Lexington, KY, USA) and University of Arkansas (Little Rock, AR, USA).
Stably transfected cells, which inducibly express the different SR-A constructs, were created using the Flp-In™ T-REx™ HEK-293 system (Invitrogen, Eugene, OR, USA), in which a single gene copy is inserted under the control of a tetracycline-inducible promoter and receptor expression induced with tetracycline (0.5 mg/ml), as described previously [16]. For culture in suspension, HEK cells were detached with trypsin, resuspended in DMEM/FBS, and cultured in ultra-low adherent polystyrene six-well plates (Corning Costar, Corning, NY, USA). To reduce aggregation, suspended cells were washed in DMEM containing EDTA (10 mM) and then filtered through cell strainers (40 μm nylon) before use in adhesion assays. Trypan blue exclusion was assessed to confirm that none of the treatments altered cell viability.
MDA-BSA
MDA-BSA was prepared using MDA bis(dimethyl acetal) as described previously [14]. Protein modification was confirmed by immunoblotting with anti-MDA-specific antibody (Academy BioMedical Co., Houston, TX, USA).
Determination of surface SR-A
MPM (106 cells/ml) or induced HEK cells (0.5×106 cells/ml) were pretreated in suspension with wortmannin (200 nM; 30 min). Treated cells were allowed to adhere to tissue-culture plates for 2 h at 37°C, and then, nonadhered cells were removed by washing with ice-cold PBS. Cell surface SR-A was then quantified by biotinylating surface proteins [16] or by flow cytometry. For biotinylation, cell surface proteins were biotinylated by incubating cells (30 min, 4°C) with EZ-Link sulfo-NHS-LC-Biotin (1 mg/ml in PBS; Thermo Scientific, Rockford, IL, USA), cells were washed, and cell lysates were prepared in MBST/OG buffer containing protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO, USA). Biotinylated (surface) proteins were precipitated from 50 μg lysate protein using streptavidin-coated beads (Sigma Chemical Co.). Cell surface (bead pellet) and total lysate proteins were resolved by SDS-PAGE (10% Bis-Tris gel, BioRad, Hercules, CA, USA), and SR-A was detected by immunoblotting with a goat polyclonal antibody to murine SR-A (R&D Systems, Minneapolis, MN, USA). Quantitative isolation of biotinylated protein was confirmed by blotting supernatant protein with streptavidin-coupled HRP. To quantify surface SR-A by flow cytometry, macrophages were suspended in ice-cold PBS containing 1% BSA and incubated with Alexa488-conjugated 2F8 mAb (30 min, 4°C). Cells were then washed and cell-associated fluorescence quantified by flow cytometry.
Cell adhesion assays
Microscopic assessment of SR-A-mediated cell adhesion was performed as described previously [15]. Briefly, induced HEK cells were pretreated in suspension with wortmannin (200 nM) or LY294002 (50 μM) for 30 min and then plated (3×104 cells/well) into four-chambered LAB-TEK slides (Nalge Nunc International, Naperville, IL, USA), precoated with the SR-A ligand MDA-BSA for 10 or 120 min. Adhered cells were washed gently with warm PBS, fixed with paraformaldehyde (4% w/v), and permeabilized with 0.1% Triton X-100. The cells were then blocked with 1% BSA for 30 min before staining with Alexa-Fluor568-conjugated phalloidin and DAPI (Invitrogen). Random fields containing ∼20 cells each were digitally imaged. Individual cells were outlined and total cell area quantified using Metamorph® or Axiovision® software.
SR-A-mediated cell attachment was assessed as described previously [16]. Briefly, induced HEK cells were pretreated in suspension with wortmannin (200 nM; 30 min), plated in 96-well plates (2×104 cells/well), and incubated at 37°C for 30 min. Adhered cells were incubated in EDTA solution (0.2 g/ml, 37°C; 10 min) to eliminate divalent cation-dependent adhesion and washed with PBS (three times, 37°C), and the remaining adhered cells were quantified using the CyQUANT™ assay (Invitrogen) according to the manufacturer’s instructions.
AcLDL association
Induced HEK cells were preincubated for 2 h in serum-free DMEM, Alexa-Fluor488-AcLDL (2.5 μg/mL, Invitrogen) was added to the media, and incubation continued for 2 h. Unbound lipoprotein was removed by washing cells with PBS, and cell-associated Alexa-Fluor488-AcLDL fluorescence was determined by flow cytometry as described previously [13]. To determine nonspecific AcLDL association, the SR-A antagonist fucoidin (75 μg/mL) was added 10 min prior to addition of Alexa-Fluor488-AcLDL.
PI3K activation
Induced HEK cells were kept in suspension or allowed to adhere to tissue-culture plates for 30 or 120 min at 37°C. To minimize the aggregation of suspended cells, methylcellulose (0.8% wt/vol) was included in the culture medium during induction. Cell lysates were prepared using ice-cold MBST/OG buffer containing phosphatase and protease inhibitors and proteins resolved by 12% Bis-Tris gel SDS-PAGE. As an index of PI3K activation, Akt phosphorylation in cell lysates was assessed by immunoblotting with phospho-specific Ser473-Akt antibody and total Akt antibody (Cell Signaling Technology, Beverly, MA, USA) [14].
Statistical analysis
Experiments were repeated at least three times and analyzed using GraphPad Prism. For Western blot and flow cytometry, results were normalized to an internal experimental control value, and significant differences of experimental treatment groups from control value were determined by one-sample t-test using GraphPad Prism. Results of morphometric analysis and cell-attachment assays were compared with control values using one-way ANOVA and Dunnett’s multiple comparison post-hoc test. Values with P < 0.05 were considered to be statistically significant.
RESULTS AND DISCUSSION
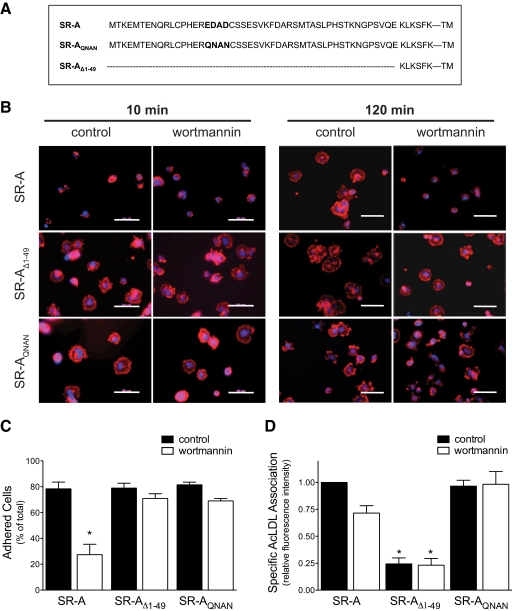
The cellular function of surface receptors is often determined by the presence of cytosolic motifs and the specific interaction of these motifs with intracellular proteins (reviewed in ref. [19]). For example, the importance of tyrosine-containing sorting motifs (e.g., NPXY, YXXØ) in clathrin-coated, pit-mediated endocytosis of the LDL, transferrin, and mannose-6-phosphate receptors has been well described [20,21,22]. In contrast, cytosolic motifs involved in regulating SR-A function are not well defined. Morimoto et al. [9] identified a VKFD sequence at residues 21–24 in the cytoplasmic tail of SR-A as a potential internalization motif by transiently expressing a series of N-terminal mutations of human SR-A in COS-7 cells. Additionally, we showed that the 6 membrane-proximal aa of murine SR-A are necessary and sufficient to mediate cell adhesion but not ligand internalization [16]. Analysis of the aa sequence of the cytoplasmic tail of SR-A from different species identified a highly conserved acidic domain (EDAD) that reportedly interacts with the cytoplasmic protein Hook3 during the endocytic sorting process [12]. To examine the potential importance of this motif in SR-A-mediated cell adhesion, the acidic aa in the EDAD motif of the murine SR-A sequence were mutated to their cognate amide derivatives (SR-AQNAN), a change that eliminated the acidic residues and maintained the predicted secondary structure and solvent exposure of this region [23]. The function and regulation of the mutated murine receptor were then studied in transfected HEK cells. The aa sequences of the N-terminal cytoplasmic tail of the full-length SR-A and the different SR-A constructs used in these studies are shown in Figure 1A.
Figure 1.
An acidic aa motif is required for PI3K-dependent, SR-A-mediated cell adhesion but not ligand uptake. (A) The aa sequence of the SR-A cytoplasmic tail for SR-A constructs (SR-A, SR-AQNAN, and SR-AΔ1–49) used in this study. (B and C) Cell attachment and spreading assays of HEK cells expressing different SR-A constructs and pretreatment with wortmannin (PI3K inhibitor) were performed as described in Materials and Methods. Cell morphology (B) was assessed by staining with Alexa-Fluor568-conjugated phalloidin (red) and DAPI (blue). Images (40× original) are representative of at least five independent experiments. The original scale bars represent 50 μm. Cell attachment (C) was determined using the CyQuant assay. The number of cells adhered was quantified and expressed as a percentage of total cells plated. Values are expressed as mean ± sem; n = 4; *, P < 0.05, compared with control SR-A values. (D) SR-A-mediated ligand uptake was assessed as described in Materials and Methods. The means ± sem of three individual experiments are shown. *, P < 0.05, compared with SR-A control values.
Cell adhesion involves enhanced cell attachment and cell spreading. Previous results have demonstrated that PI3K is activated during SR-A-mediated cell adhesion and that inhibiting PI3K abolishes the ability of SR-A to enhance cell spreading [14]. To define the importance of the intracellular domain of the receptor for PI3K-sensitive SR-A-mediated cell adhesion, we examined the adhesion of stably transfected HEK cells that inducibly express wild-type SR-A, a truncated receptor; SR-AΔ1–49, which lacks all but the 6 membrane-proximal cytoplasmic aa [16]; or SR-AQNAN. Briefly, SR-A-, SR-AΔ1–49-, and SR-AQNAN-expressing cells were pretreated with PI3K inhibitor, wortmannin, or LY294002 or vehicle control and then allowed to adhere to slides coated with the SR-A ligand MDA-BSA for 10 or 120 min. Adhered cells were then fixed and stained with fluorescently conjugated phalloidin and cell adhesion assessed by quantifying cell surface area. As shown in Figure 1B and quantified in Table 1, cells expressing SR-AΔ1–49 and SR-AQNAN showed greater spreading at 10 min than those expressing wild-type SR-A. However, at 120 min SR-A, there was no difference in the spreading of SR-AΔ1–49- and SR-AQNAN-expressing cells, indicating that deletion of the acidic EDAD domain enhances the rate of cell spreading. Pretreating cells with wortmannin (Fig. 1 and Table 1) or LY294002 (Table 1) eliminated the enhanced spreading of SR-A-expressing cells, indicating a requirement for PI3K activation. In contrast, the enhanced spreading of SR-AΔ1–49- or SR-AQNAN-expressing cells was not affected by inhibiting PI3K, even at an early time-point (10 min). Thus, deletion of the EDAD motif increased the rate and removed the PI3K sensitivity of SR-A-mediated cell adhesion.
TABLE 1.
Dependency of SR-A-Mediated Cell Spreading on PI3K
| 10 min
|
120 min
|
|||||
|---|---|---|---|---|---|---|
| Control | Wortmannin | LY294002 | Control | Wortmannin | LY294002 | |
| Cell surface area (μm2) | Cell surface area (μm2) | |||||
| SR-A | 130.1 ± 16.8 | 119.3 ± 4.9 | 130.1 ± 16.8 | 705.6 ± 27.7 | 197.4 ± 16.3a | 158.8 ± 11.4a |
| SR-AΔ1–49 | 358.3 ± 27.3a | 362.7 ± 8.4a | 336.5 ± 26.9a | 751.7 ± 59.6 | 735.6 ± 45.0 | 719.3 ± 19.4 |
| SR-AQNAN | 442.0 ± 52.1a | 434.9 ± 21.6a | 412.6 ± 40.9a | 701.5 ± 45.8 | 773.8 ± 44.1 | 728.5 ± 13.7 |
Cell surface area was assessed using Alexa-Fluor568-phalloidin to stain cells and quantified using image analysis software. Data represent the mean ± sem obtained from images representing at least three independent experiments.
P < 0.05 compared with the untreated SR-A control value for that time.
As a second index of cell adhesion, the ability of SR-A, SR-AΔ1–49, and SR-AQNAN to increase the attachment of transfected HEK cells to tissue-culture plates was assessed. In these experiments, cells expressing the receptor constructs were pretreated with wortmannin or vehicle control, plated in 96-well plates for 30 min, washed to remove nonadhered cells, and the cells that remained attached quantified using the CyQuant™ assay. As shown in Figure 1C, inhibiting PI3K activation reduced the number of attached SR-A-expressing cells. In contrast, the attachment of SR-AΔ1–49- and SR-AQNAN-expressing cells was not affected by PI3K inhibition. These results are consistent with the observed morphologic changes and indicate that a motif, EDAD, within the 1–49 region of the cytoplasmic domain is specifically involved in the PI3K-dependent regulation of SR-A-mediated cell adhesion.
To assess the importance of the acidic EDAD motif in regulating SR-A-mediated ligand uptake, SR-A-, SR-AΔ1–49-, and SR-AQNAN-expressing cells were pretreated with wortmannin prior to incubating with fluorescently labeled AcLDL, a specific ligand for SR-A. As shown in Figure 1D, expression of SR-A and SR-AQNAN enhanced association of fluorescently labeled AcLDL with cells to a similar extent. This result contrasts with a recent report that changing the acidic aa in the EDAD motif of the human SR-A sequence to alanine alters ligand internalization [12]. The difference may reflect the different mutations used or the level of receptor expression achieved in transfected cells. As reported previously [16], SR-AΔ1–49 did not enhance AcLDL uptake in transfected cells. Inhibiting PI3K with wortmannin did not affect AcLDL association significantly (P>0.05) in cells expressing any of the SR-A constructs. Thus, SR-A-mediated ligand uptake is not affected by mutation of the acidic aa motif and in contrast to cell adhesion, does not require PI3K activation.
The above results demonstrate an important regulatory role for an acidic motif in the cytoplasmic tail of SR-A. Several possible mechanisms were examined to define the importance of this motif. First, we examined the possibility that eliminating the acidic motif decreased receptor expression. Incubating transfected HEK cells with tetracycline induced similar expression of RNA for each receptor (data not shown). Further, as shown in Figure 2A, the wild-type SR-A and SR-AQNAN proteins were expressed and formed multimeric complexes to a similar extent. As reported previously, expression of the SR-AΔ1–49 protein was greater than that of wild-type SR-A, presumably as a result of increased stability resulting from a lack of receptor internalization [16]. We next examined the possibility that the receptors might differ in their ability to couple to downstream signals required for cell adhesion. Previous results demonstrated that PI3K is activated during SR-A-mediated macrophage adhesion [14]. Therefore, we examined the ability of SR-A, SR-AΔ1–49, and SR-AQNAN to activate PI3K during cell adhesion. For this, cells expressing the receptor constructs were kept in suspension (no adhesion) or allowed to adhere for 30 or 120 min. Phosphorylation of Akt in cell lysates was measured as an index of PI3K activation. Results presented in Figure 2B indicate that PI3K activation during adhesion is similar in cells expressing the different SR-A constructs. Thus, differences in expression, formation of oligomeric complexes, or the coupling of the receptor to PI3K activation do not account for the differences in function of the different SR-A constructs.
Figure 2.
Expression and PI3K activation during adhesion of transfected HEK-293 cells. Transfected HEK cells were cultured in suspension with tetracycline (0.5 μg/ml, 16 h) to induce SR-A expression. (A) Induced cells were adhered for 120 min, cell lysates were prepared, and proteins were resolved by SDS-PAGE under reducing and nonreducing conditions and then immunoblotted with a SR-A antibody. The arrows indicate the monomeric (≈70 kDa), dimeric, and trimeric forms of the receptor. (B) Induced cells were kept in suspension (0 min; susp) or allowed to adhere for 30 or 120 min. Cell lysates were prepared, and proteins were resolved by SDS-PAGE and immunoblotted for phospho-S473Akt (pAkt) and total Akt (tAkt). Blots are representative of at least three independent experiments.
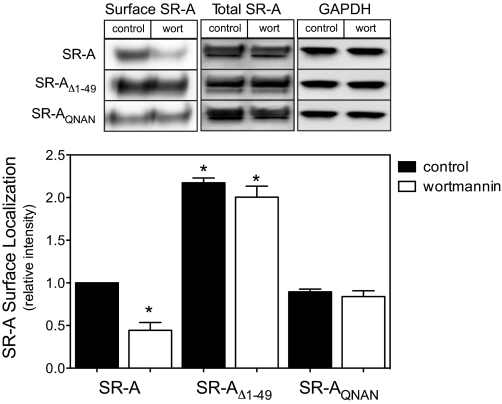
Modulating receptor localization on the cell surface represents an important mechanism for regulating receptor function, and several studies support a role for PI3K activation in receptor trafficking from an intracellular pool to the plasma membrane [24,25,26]. To assess the role of PI3K in regulating SR-A localization during cell adhesion, SR-A-, SR-AΔ1–49-, and SR-AQNAN-expressing cells were treated with PI3K inhibitors prior to cell adhesion for 2 h. The plasma membrane localization of the different SR-A constructs was then assessed by labeling membrane proteins with NHS-biotin at 4°C (to prevent biotin internalization and the potential labeling of intracellular proteins), excess biotin was removed by washing, and protein lysates were prepared. Surface (i.e., biotinylated) proteins were then isolated using streptavidin-coupled beads, resolved by SDS-PAGE, and immunoblotted with a SR-A-specific antibody. As shown in Figure 3, the amount of SR-AQNAN and SR-A detected on the cell surface was similar. Consistent with its inability to internalize ligand, SR-AΔ1–49 localization on the cell surface was higher than that of SR-A or SR-AQNAN. Pretreating cells with wortmannin reduced the amount of SR-A on the cell surface to 50% of untreated cells without affecting total receptor expression. In contrast, inhibiting PI3K did not affect the cell surface localization of SR-AΔ1–49 or SR-AQNAN. Similar results were obtained using another PI3K inhibitor, LY294002 (50 μM; not shown). This finding indicates that the acidic aa in a cytoplasmic EDAD motif are required specifically for PI3K-dependent regulation of SR-A cell surface localization.
Figure 3.
An acidic aa motif is required for PI3K-dependent SR-A cell surface localization. Following SR-A induction and treatment with PI3K inhibitors in suspension, cells were allowed to adhere for 2 h. Cell surface proteins were biotinylated and precipitated from cell lysates using streptavidin-coated beads as described in Materials and Methods. The amount of precipitated (Surface) SR-A and total SR-A in cell lysates was assessed via immunoblotting with antibodies specific for SR-A. The intensities of surface SR-A were normalized to that detected in control SR-A-expressing cells. The amount of GAPDH in cell lysates was blotted as a control. Results obtained from a representative experiment and the mean ± sem of three separate experiments are shown. *, P < 0.05, compared with control SR-A values.
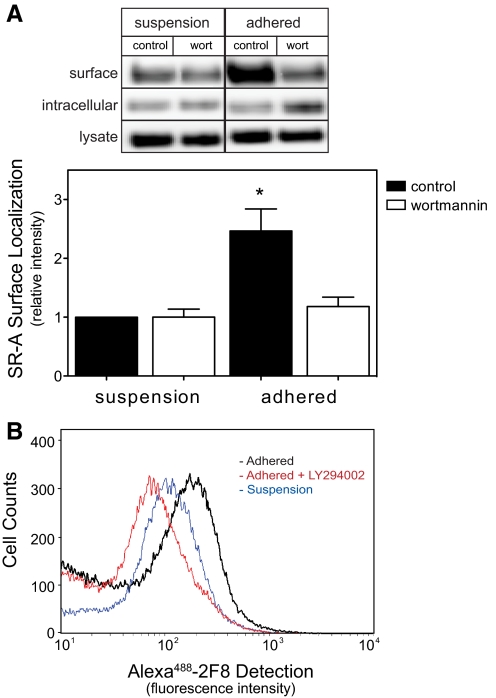
The finding that PI3K regulates SR-A localization has not been reported previously but may represent an important cellular mechanism for regulating SR-A function in macrophages. Therefore, we examined SR-A localization in isolated peritoneal macrophages, which endogenously express SR-A. Using a protocol analogous to that used for HEK cells, isolated peritoneal macrophages were treated with PI3K inhibitors and then kept in suspension or adhered to tissue-culture plates for 2 h. The amount of cell surface SR-A was then quantified as described above for HEK cells. As shown in Figure 4A, macrophage adhesion increased the amount of SR-A detected on the cell surface. Pretreating cells with wortmannin abolished the translocation of SR-A to the cell surface stimulated by macrophage adhesion but did not affect total SR-A expression. The PI3K-dependent translocation of SR-A to the cell surface during macrophage adhesion was confirmed using flow cytometry and LY294002 (50 μM) to inhibit PI3K (Fig. 4B). Together, these results demonstrate that SR-A-mediated cell adhesion induces a translocation of intracellular SR-A to the cell surface by activating a PI3K-dependent pathway.
Figure 4.
PI3K activation increases SR-A surface localization during macrophage adhesion. MPM were isolated, treated in suspension with wortmannin, and then kept in suspension or allowed to adhere for 2 h. (A) Cell surface proteins were biotinylated and precipitated from lysates as described for Figure 3. The amount SR-A present in the original lysate, streptavidin precipitate (surface), or supernatant (intracellular) was assessed via immunoblotting with antibodies specific for SR-A and the intensities normalized to that detected in control macrophages kept in suspension. Results obtained from a representative experiment and the mean ± sem of four separate experiments are shown. *, P < 0.05, compared with control suspension values. (B) Alternatively, the amount of SR-A on the cell surface was determined labeling surface receptors with Alexa488-conjugated 2F8 mAb (30 min, 4°C) and cell-associated fluorescence quantified by flow cytometry. Data from a representative experiment are shown. The blue tracing indicates 2F8 association with macrophages in suspension, the black tracing indicates 2F8 association following 120 min of adhesion, and the red tracing indicates 2F8 association with LY294002-treated macrophages that were adhered for 120 min.
Macrophages play important roles in innate and acquired immunity, inflammation, and tissue remodeling. The ability of macrophages to participate in such processes depends on the recognition of extracellular ligands by cell surface receptors involved in macrophage migration, adhesion, and ligand internalization. SR-A recognizes a variety of such ligands, and their importance in many pathologic processes (e.g., atherosclerosis, host defense, Alzheimer’s disease, and diabetes) has been suggested [2, 6, 27, 28]. Results of several studies indicate that SR-A-mediated ligand uptake reduces the inflammatory response of macrophages [29, 30]. In contrast, relatively few studies have addressed the physiological significance of SR-A-mediated macrophage adhesion. However, recent studies suggest that SR-A-mediated macrophage adhesion may enhance an inflammatory response [7, 27, 28]. Taken together, the currently available data suggest that SR-A-mediated ligand uptake and macrophage adhesion have opposing effects on inflammatory processes and that there may be a benefit in selectively inhibiting one or the other functions. Thus, our findings indicating that cytoplasmic motifs of SR-A are differentially involved in the regulation of SR-A function by PI3K have important implications in diverse inflammatory settings, where SR-A ligands are thought to accumulate.
In summary, our results show that mutating (SR-AQNAN) or deleting (SR-AΔ1–49) a cytosolic acidic motif (EDAD) abolishes the PI3K dependency of SR-A-mediated cell adhesion without affecting SR-A-mediated ligand uptake. These results identify an acidic motif that is involved specifically in the regulation of SR-A-mediated cell adhesion and underscore the divergent structural requirements for regulating SR-A function. That SR-A trafficking is similarly regulated is indicated by our results showing that PI3K activation during SR-A-mediated cell adhesion enhances SR-A localization on the cell surface. Further, we show that removal of an EDAD motif by deletion (SR-AΔ1–49) or mutation (SR-AQNAN) abrogates the requirement for PI3K activation in translocating SR-A to the cell surface, suggesting a novel role for this motif in regulating SR-A trafficking. Together, our results suggest a pathway in which SR-A-mediated adhesion to a modified protein activates PI3K, which in turn, recruits more SR-A to the cell surface in a positive-feedback mechanism to enhance cell adhesion further.
AUTHORSHIP
J. C. and D. N. contributed equally to performing and analyzing experiments and in preparing the manuscript. S. R. P. designed experiments, interpreted results, and prepared the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (RO1HL88588 and RO1HL075241) and an Established Investigator Award from the American Heart Association (S. R. P.). J. C. was supported by a NIH training grant (DK07778). The authors acknowledge Cecelia Gass and Stuart Rice for their technical assistance, Dr. Ginell Post for the critical review, and members of the Cardiovascular Research Center and the Kentucky Pediatrics Research Institute at the University of Kentucky for their helpful comments and suggestions.
Footnotes
Abbreviations: AcLDL=acetylated low-density lipoprotein, DAPI=4′,6-diamidino-2-phenylindole, HEK=human embryo kidney, MBST/OG=25 mM morpholine-ethanesulfonic acid, 150 mM NaCl, 1% Triton X-100, pH 6.4/60 mM octylglucopyranoside, MDA-BSA=malondialdehyde-modified BSA, MPM=mouse peritoneal macrophage(s), NHS=normal human serum, NIH=National Institutes of Health, SR-A=class A scavenger receptor(s)
References
- Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Platt N, Haworth R, Darley L, Gordon S. The many roles of the class A macrophage scavenger receptor. Int Rev Cytol. 2002;212:1–40. doi: 10.1016/s0074-7696(01)12002-4. [DOI] [PubMed] [Google Scholar]
- Gowen B B, Borg T K, Ghaffar A, Mayer E P. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol. 2000;19:61–71. doi: 10.1016/s0945-053x(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Gowen B B, Borg T K, Ghaffar A, Mayer E P. The collagenous domain of class A scavenger receptors is involved in macrophage adhesion to collagens. J Leukoc Biol. 2001;69:575–582. [PubMed] [Google Scholar]
- Santiago-Garcia J, Kodama T, Pitas R E. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem. 2003;278:6942–6946. doi: 10.1074/jbc.M208358200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kruijt J K, van Berkel T J C, Steinbrecher U P, Ishibashi S, Maeda N, Gordon S, Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Kosswig N, Cornicelli J A, Whitman S C, Wolle S, Rateri D L. Macrophage-specific expression of class A scavenger receptors enhances granuloma formation in the absence of increased lipid deposition. J Lipid Res. 2001;42:1049–1055. [PubMed] [Google Scholar]
- Van Velzen A G, Suzuki H, Kodama T, van Berkel T J. The role of scavenger receptor class A in the adhesion of cells is dependent on cell type and cellular activation state. Exp Cell Res. 1999;250:264–271. doi: 10.1006/excr.1999.4530. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Wada Y, Hinagata J, Imanishi T, Kodama T, Doi T. VXFD in the cytoplasmic domain of macrophage scavenger receptors mediates their efficient internalization and cell-surface expression. Biol Pharm Bull. 1999;22:1022–1026. doi: 10.1248/bpb.22.1022. [DOI] [PubMed] [Google Scholar]
- Whitman S C, Daugherty A, Post S R. Regulation of acetylated low density lipoprotein uptake in macrophages by pertussis toxin-sensitive G proteins. J Lipid Res. 2000;41:807–813. [PubMed] [Google Scholar]
- Fong L G, Le D. The processing of ligands by the class A scavenger receptor is dependent on signal information located in the cytoplasmic domain. J Biol Chem. 1999;274:36808–36816. doi: 10.1074/jbc.274.51.36808. [DOI] [PubMed] [Google Scholar]
- Sano H, Ishino M, Kramer H, Shimizu T, Mitsuzawa H, Nishitani C, Kuroki Y. The microtubule-binding protein Hook3 interacts with a cytoplasmic domain of scavenger receptor A. J Biol Chem. 2007;282:7973–7981. doi: 10.1074/jbc.M611537200. [DOI] [PubMed] [Google Scholar]
- Post S R, Gass C, Rice S, Nikolic D, Crump H, Post G R. Class A scavenger receptors mediate cell adhesion via activation of G(i/o) and formation of focal adhesion complexes. J Lipid Res. 2002;43:1829–1836. doi: 10.1194/jlr.m200231-jlr200. [DOI] [PubMed] [Google Scholar]
- Nikolic D M, Cholewa J, Gass C, Gong M C, Post S R. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292:C1450–C1458. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- Nikolic D M, Gong M C, Turk J, Post S R. Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A(2) and 12/15-lipoxygenase to Rac and Cdc42 activation. J Biol Chem. 2007;282:33405–33411. doi: 10.1074/jbc.M704133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosswig N, Rice S, Daugherty A, Post S R. Class A scavenger receptor-mediated adhesion and internalization require distinct cytoplasmic domains. J Biol Chem. 2003;278:34219–34225. doi: 10.1074/jbc.M303465200. [DOI] [PubMed] [Google Scholar]
- Berton G, Lowell C A. Integrin signaling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- DeMali K A, Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci. 2003;116:2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- Bonifacino J S, Traub L M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Chen W J, Goldstein J L, Brown M S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Canfield W M, Johnson K F, Ye R D, Gregory W, Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24–29 of the cytoplasmic tail. J Biol Chem. 1991;266:5682–5688. [PubMed] [Google Scholar]
- Collawn J F, Stangel M, Kuhn L A, Esekogwu V, Jing S Q, Trowbridge I S, Tainer J A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Clarke J F, Ester C J, Young P W, Kasuga M, Holman G D. Phosphatidylinositol 3-kinase acts at an intracellular membrane site to enhance GLUT4 exocytosis in 3T3–L1 cells. Biochem J. 1996;313:125–131. doi: 10.1042/bj3130125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Eckhardt E R, Post S R, van der Westhuyzen D R. Phosphatidylinositol-3-kinase regulates scavenger receptor class B type I subcellular localization and selective lipid uptake in hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:2125–2131. doi: 10.1161/01.ATV.0000233335.26362.37. [DOI] [PubMed] [Google Scholar]
- Du L, Post S R. Macrophage colony-stimulating factor differentially regulates low density lipoprotein and transferrin receptors. J Lipid Res. 2004;45:1733–1740. doi: 10.1194/jlr.M400140-JLR200. [DOI] [PubMed] [Google Scholar]
- Usui H K, Shikata K, Sasaki M, Okada S, Matsuda M, Shikata Y, Ogawa D, Kido Y, Nagase R, Yozai K, Ohga S, Tone A, Wada J, Takeya M, Horiuchi S, Kodama T, Makino H. Macrophage scavenger receptor-A-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56:363–372. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman S E, Thomas C A, Cao L, Silverstein S C, Loike J D. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J Immunol. 2004;173:6427–6432. doi: 10.4049/jimmunol.173.10.6427. [DOI] [PubMed] [Google Scholar]
- Pluddemann A, Hoe J C, Makepeace K, Moxon E R, Gordon S. The macrophage scavenger receptor a is host-protective in experimental meningococcal septicemia. PLoS Pathog. 2009;5:e1000297. doi: 10.1371/journal.ppat.1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]