Fig. 2.
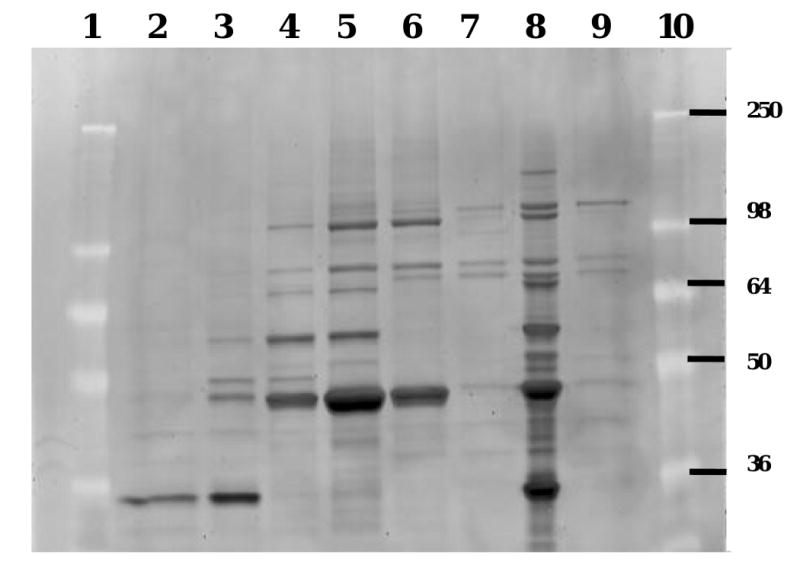
Superdex-200 purification of puromycin-sensitive aminopeptidase from Caco-2 cell homogenates. Active and non-active fractions from the Superdex-200 purification were analyzed by 10% SDS-PAGE, here stained with SYPRO Red. Lanes 4-6 contain active fractions hydrolyzing Val-Ser-cHPMPC, while lanes 2, 3, 7 and 9 are non-active fractions. Lane 8 corresponds to the pooled active MonoQ fractions that were initially applied to the Superdex-200 column. Lanes 1 and 10 are size markers with molecular mass expressed in kDa. The band visible at ∼ 100 kDa in lane 4-6 and 8 was exclusively present in the active fractions and its identity was determined by tandem mass spectrometry and database searches to be puromycin-sensitive aminopeptidase (APP-S).
