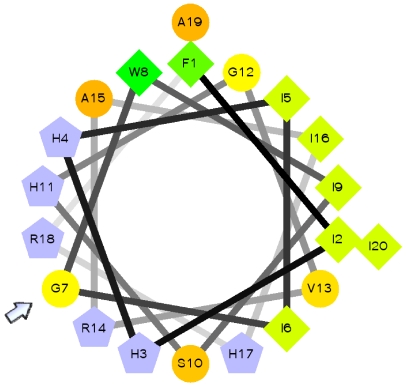
Figure 4. Amphipathic α-helical structure of cod piscidin.
Schiffer-Edmundson helical wheel projection of piscidin from Gadus morhua, showing that it is predicted to adopt an almost perfect amphipathic structure. The amphipathicity of the helix is disrupted by glycine at position 7 (arrow), which corresponds to a positively selected site. Hydrophilic charged and very hydrophobic residues are represented by grey pentagons and green diamonds, respectively. The circles denote other neutral or polar amino acids. Residues are numbered starting from the amino terminus of the mature peptides.