Abstract
The primary immunization of outbred mice with the antigen Pap-S-DNPL results in the generation of low titers of anti-DNP antibody which in about one-third of the responding animals is as homogeneous as a myeloma protein by the combined criteria of (a) isoelectric focusing in gels and (b) gel electrophoresis of the antibody light chains. The electrophoretically homogeneous antibodies show a marked restriction of isoelectric points near pH 5.0. Such marked selectivity of the antihapten antibodies appears to result from the chemical and structural homogeneity of the Pap-S-DNPL antigen.
Full text
PDF

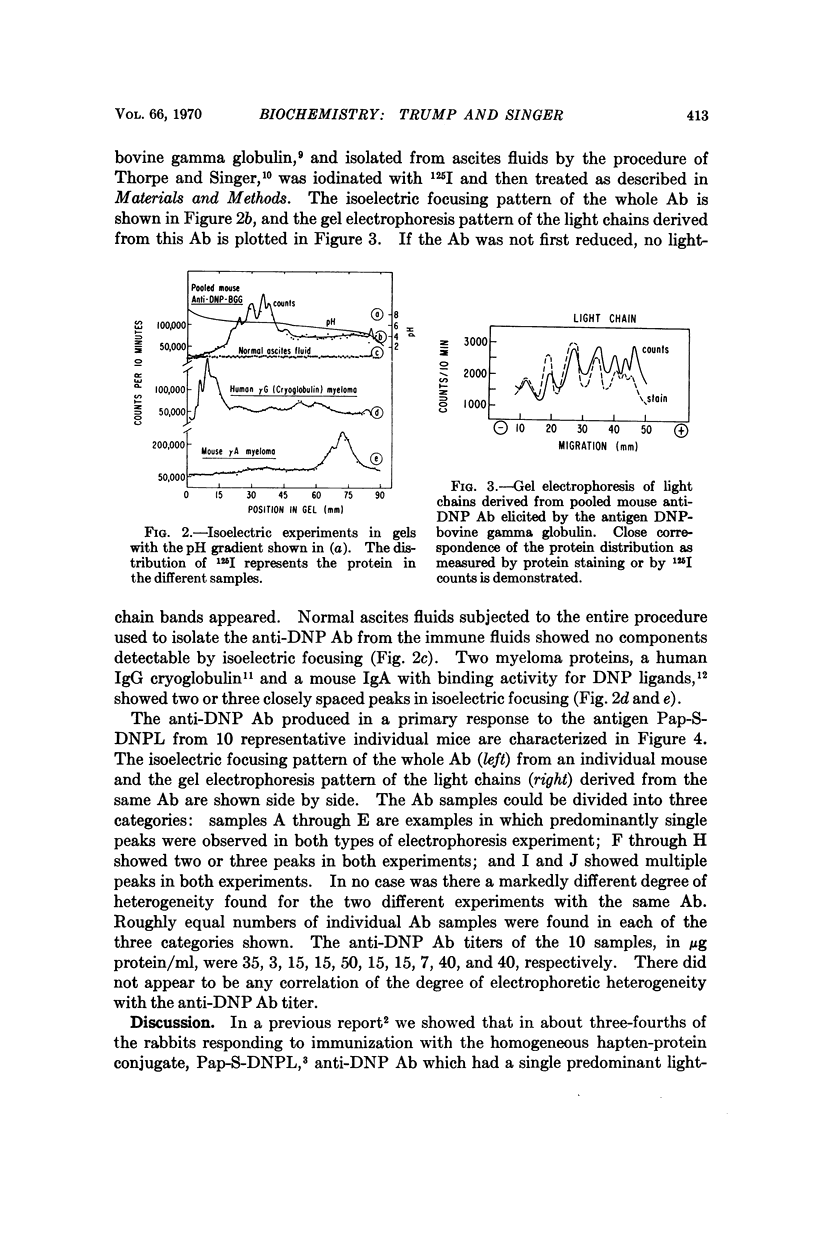
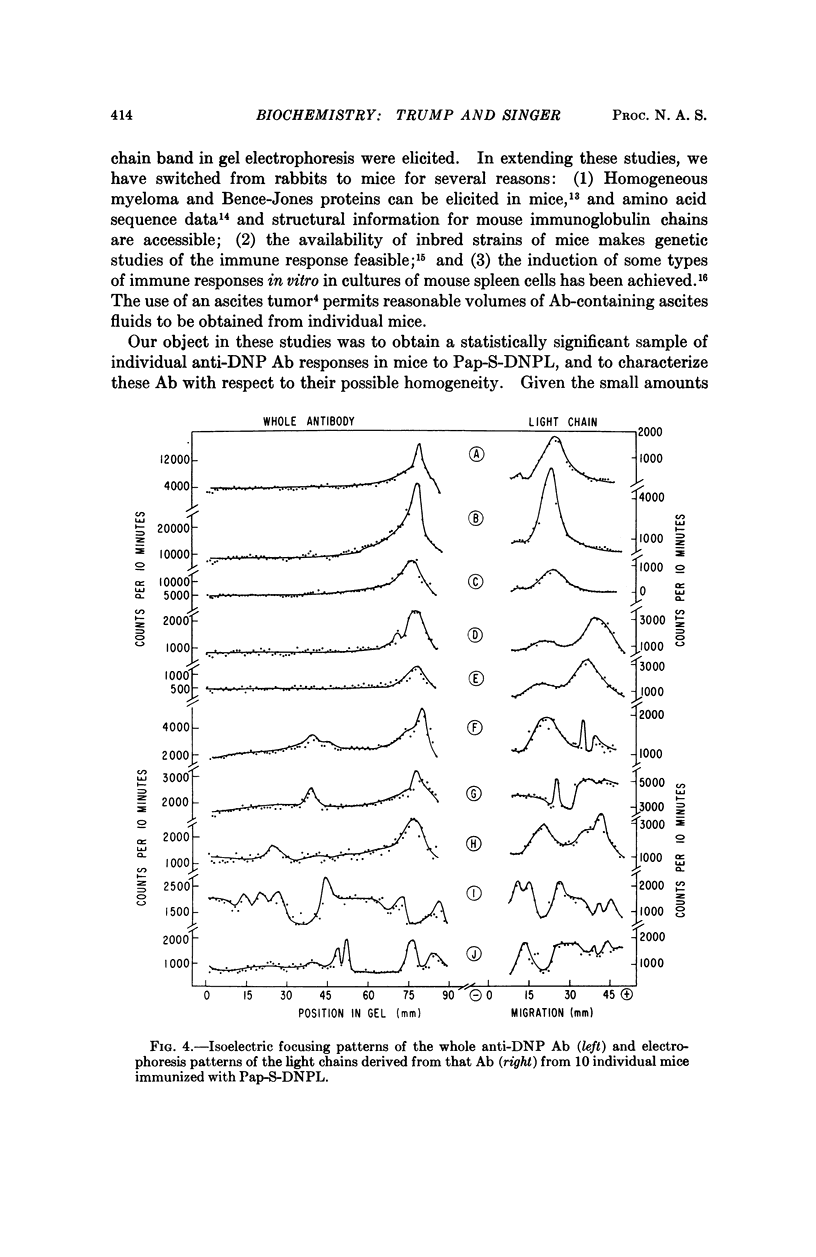




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Braun D. G., Eichmann K., Krause R. M. Rabbit antibodies to streptococcal carbohydrates. Influence of primary and secondary immunization and of possible genetic factors on the antibody response. J Exp Med. 1969 Apr 1;129(4):809–830. doi: 10.1084/jem.129.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman L., Singer S. J. On homogeneous antigens and antibodies. 3. The preparation and properties of the univalent hapten-protein conjugate papain-S-DNPL. Ann N Y Acad Sci. 1970 Feb 13;169(1):72–92. doi: 10.1111/j.1749-6632.1970.tb55972.x. [DOI] [PubMed] [Google Scholar]
- Brenneman L., Singer S. J. The generation of antihapten antibodies with electrophoretically homogeneous L chains. Proc Natl Acad Sci U S A. 1968 May;60(1):258–264. doi: 10.1073/pnas.60.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Porter R. R. Heterogeneity of the peptide chains of gamma-globulin. Biochem J. 1964 Feb;90(2):278–284. doi: 10.1042/bj0900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G., Latner A. L. Isoelectric focusing in polyacrylamide gels. Lancet. 1968 Apr 20;1(7547):847–848. doi: 10.1016/s0140-6736(68)90303-6. [DOI] [PubMed] [Google Scholar]
- FARAH F. S., KERN M., EISEN H. N. The preparation and some properties of purified antibody specific for the 2,4-dinitrophenyl group. J Exp Med. 1960 Dec 1;112:1195–1210. doi: 10.1084/jem.112.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Haber E. Immunochemistry. Annu Rev Biochem. 1968;37:497–520. doi: 10.1146/annurev.bi.37.070168.002433. [DOI] [PubMed] [Google Scholar]
- Hood L., Lackland H., Eichman K., Kindt T. J., Braun D. G., Krause R. M. Amino acid sequence restriction in rabbit antibody light chains. Proc Natl Acad Sci U S A. 1969 Jul;63(3):890–896. doi: 10.1073/pnas.63.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C. A., Eisen H. N., Dray S. Distribution of allelic allotypes among gamma-G-immunoglobulins and purified anti-hapten antibodies from the same rabbit. J Immunol. 1965 Sep;95(3):404–411. [PubMed] [Google Scholar]
- Little J. R., Counts R. B. Affinity and heterogeneity of antibodies induced by epsilon-2,4-dinitrophenylinsulin. Biochemistry. 1969 Jul;8(7):2729–2736. doi: 10.1021/bi00835a006. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965 Sep 1;122(3):517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Lennox E. S., Facon M. A carbohydrate-containing mouse light chain-protein. Biochem Biophys Res Commun. 1966 Jul 20;24(2):244–251. doi: 10.1016/0006-291x(66)90727-3. [DOI] [PubMed] [Google Scholar]
- POTTER M., BOYCE C. R. Induction of plasma-cell neoplasms in strain BALB/c mice with mineral oil and mineral oil adjuvants. Nature. 1962 Mar 17;193:1086–1087. doi: 10.1038/1931086a0. [DOI] [PubMed] [Google Scholar]
- Pincus J. H., Haber E., Katz M., Pappenheimer A. M., Jr Antibodies to pneumococcal polysaccharides: relation between binding and electrophoretic heterogeneity. Science. 1968 Nov 8;162(3854):667–668. doi: 10.1126/science.162.3854.667. [DOI] [PubMed] [Google Scholar]
- Rüde E., Mozes E., Sela M. Role of the net electrical charge of the complete antigen in determining the chemical nature of anti-p-azobenzenearsonate antibodies. Biochemistry. 1968 Aug;7(8):2971–2975. doi: 10.1021/bi00848a038. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., KIMMEL J. R., BROWN D. M. Crystalline papain. II. Physical studies; the mercury complex. J Biol Chem. 1954 Apr;207(2):533–549. [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Sela M., Mozes E. Dependence of the chemical nature of antibodies on the net electrical charge of antigens. Proc Natl Acad Sci U S A. 1966 Feb;55(2):445–452. doi: 10.1073/pnas.55.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe N. O., Singer S. J. The affinity-labeled residues in antibody active sites. II. Nearest-neighbor analyses. Biochemistry. 1969 Nov;8(11):4523–4534. doi: 10.1021/bi00839a045. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Rockey J. H. Antivasopressin antibody. Characterization of high-affinity rabbit antibody with limited association constant heterogeneity. Biochemistry. 1969 Jul;8(7):2719–2728. doi: 10.1021/bi00835a005. [DOI] [PubMed] [Google Scholar]



