Abstract
Objective
We examined CD11c, a β2 integrin, on adipose tissue (AT) leukocytes and blood monocytes, and its role in diet-induced obesity.
Methods and Results
High-fat diet–induced obese C57BL/6 mice, CD11c-deficient mice, and obese humans were studied. CD11c, leukocytes, and chemokines/cytokines were examined in AT and/or blood by flow cytometry, RNase protection assay, quantitative PCR, or ELISA. Obese C57BL/6 mice had increased CD11c in AT and blood compared with lean controls. CD11c mRNA positively correlated with MCP-1 in human visceral AT. Obese humans with metabolic syndrome had higher CD11c on blood monocytes than leans. Low-fat diet–induced weight loss reduced blood monocyte CD11c in obese mice and humans. Mouse and human monocyte CD11c levels and mouse AT CD11c mRNA correlated with insulin resistance. CD11c deficiency in mice did not alter weight gain but decreased inflammation, evidenced by lower T-cell number and reduced levels of MHC class II, CCL5, CCL4, and IFN-γ in AT, and ameliorated insulin resistance and glucose intolerance associated with diet-induced obesity.
Conclusions
Diet-induced obesity increased CD11c in both AT and blood in mice and humans. CD11c plays an important role in T-cell accumulation and activation in AT, and contributes to insulin resistance associated with obesity.
Keywords: inflammation, obesity
Obesity increases risk for type 2 diabetes and cardiovascular disease (CVD). Chronic inflammation, which occurs in obesity, has been acknowledged as an important link between obesity and the development of diabetes and CVD. Adipose tissue (AT) synthesizes and secretes proinflammatory substances such as cytokines, which are up-regulated in obesity and may play important roles in mediating obesity-linked insulin resistance.1 Chemokines, which contribute to inflammation because of their active properties for leukocyte trafficking and activation, have also been shown to be expressed by AT and increased in obesity.2,3 Indeed, along with the increased levels of chemokines such as monocyte chemoattractant protein–1 (MCP-1, or CCL2), and regulated on activation, normal T cell expressed and secreted (RANTES, or CCL5), leukocytes including macrophages and T cells are increased in AT in obesity, and increased leukocytes in AT may contribute to obesity-linked metabolic abnormalities.2,4-7
Initial studies used CD11b and/or F4/80 to define total macrophages in AT.4,5 Using mice fed high-fat diet (HFD) for 3 weeks, we first reported a significant increase in CD11c+ cells in AT.8 Subsequently, other investigators reported accumulation of F4/80+CD11c+ leukocytes in AT of obese mice.9,10 CD11c+ leukocytes in AT of obese mice show proinflammatory characteristics of classically activated macrophages (M1)9,10 and were demonstrated to play an important role in obesity-linked AT inflammation and the development of insulin resistance.11
CD11c is a member of the β2 integrins and is expressed on mouse dendritic cells (DCs) and a subpopulation of mouse monocytes/macrophages.12 CD11c contributes to monocyte adhesion to inflamed endothelial cells by binding vascular cell adhesion molecule–1 (VCAM-1), and has been used as an activation marker for monocytes/macrophages.13 Blood monocyte CD11c is increased in hyperlipidemia and plays an important role in the development of atherosclerosis in apoE-deficient mice.12 Previous studies related to obesity have focused on CD11c expression in AT8-10; the systemic effect of obesity on blood monocyte CD11c expression is not known. Furthermore, despite the demonstrated role of CD11c+ cells in obesity,11 the role of CD11c in obesity-related AT inflammation and metabolic dysfunctions remains to be defined.
In the present study, using mice fed HFD for 6 months and obese humans with metabolic syndrome (MS), we examined CD11c expression in blood and AT, and the role of CD11c in AT inflammation.
Materials and Methods
Detailed methods can be found in the online-only Data Supplement (please see www.ahajournals.org).
Animal models
Male CD11c−/−12 and C57BL/6 wild-type (WT) mice were used. Obesity was induced by HFD (21% w/w fat [41% of kcal from fat]; Dyets Inc., Bethlehem, PA), with mice fed normal diet (ND; 4.5% w/w fat [12% of kcal from fat], PicoLab Rodent Chow 5053) used as lean controls.2 Some of the obese mice underwent weight reduction by switching from HFD to ND and being fed ND ad libitum for an additional 4 weeks. All animal studies were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine, and all experimental procedures were in accordance with institutional guidelines.
Human studies
Human visceral AT (VAT; perigastric omentum) was collected from 21 morbidly obese patients (1 male, 20 females) at the time of bariatric surgery. Additional obese individuals (1 male, 8 females) who met the criteria of MS14,15 and gender- and age-matched lean healthy controls (online Table I; please see www.ahajournals.org) were recruited. Weight reduction in obese patients with MS was induced by a protein-sparing, very-low-calorie diet as previously reported.3 Blood was taken at baseline (obese and healthy subjects) and after 4–6 weeks of weight loss (obese subjects) to examine monocyte CD11c expression. All human studies were approved by the Institutional Review Board of Baylor College of Medicine, and informed consent was obtained.
AT fractionation and flow cytometric (FACS) analysis
Collagenase digestion was performed to fractionate AT into adipocytes and stromal/vascular cells (S/Vs). FACS analysis was performed to detect CD11c on mouse AT S/Vs, or mouse or human blood monocytes, or to examine leukocytes in mouse AT.12
Quantitation of mRNA and protein
Chemokines/cytokines or macrophage and T-cell markers were examined by RNase protection assay (RPA) or quantitative reverse transcriptase polymerase chain reaction (RT-PCR) for mRNA, and Quantikine enzyme-linked immunosorbent assay (ELISA) for proteins.2
Biochemical measurements and glucose tolerance test in mice
Fasting plasma levels of glucose and insulin were measured, and insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) using the formula: fasting insulin (μIU/ml) × fasting glucose (mmol/l)/22.5. Glucose tolerance test (GTT) was performed in mice after an overnight fast. Blood glucose concentrations were measured with a Glucometer Elite XL blood glucose meter (Bayer Corporation) before and 15, 30, 45, 60, 90, and 120 minutes after an intraperitoneal injection of dextrose dissolved in water (1 g/kg).
Statistical analysis
Values are presented as mean±SEM. Student's t-test (for comparison between 2 groups) or one-way ANOVA (for comparisons of 3 or more groups) followed by Bonferroni multiple comparison test was used for statistical analysis; Spearman correlation coefficents were computed to examine correlations. Differences were considered significant at P≤0.05.
Results
CD11c+ leukocytes in mouse AT
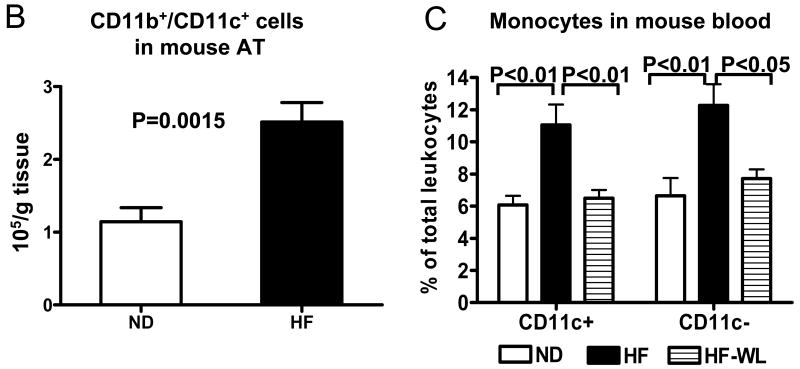
Using FACS analysis, we examined CD11b+/CD11c+ and CD11b+/CD11c− cells in S/Vs of mouse AT. CD11b+/CD11c+ and CD11b+/CD11c− cells both expressed F4/80, but did not express CD205 (Figure 1A). However, CD11b+/CD11c+ cells expressed higher levels of MHC class II than CD11b+/CD11c− cells (Figure 1A). All CD11b+/CD11c+ leukocytes were Ly-6Clow, while a small subset of CD11b+/CD11c− leukocytes were Ly-6Chigh (Figure 1A). The numbers of CD11b+/CD11c+ leukocytes per gram of AT were significantly higher in obese mice than lean mice (Figure 1B), which was consistent with previous reports.8-10 Furthermore, obese mice had higher ratios of CD11b+/CD11c+ leukocytes in the total CD11b+ leukocyte population of S/Vs than lean mice (77±1% in obese [n=9] vs. 41±5% in lean mice [n=7], P<0.001). CD11c mRNA in mouse AT was positively correlated with HOMA-IR (r=0.49, P=0.02, n=22). Compared to lean mice, obese mice also had higher MCP-1 levels in AT (and liver) (online Figure IA, IB; please see www.ahajournals.org).
Figure 1. CD11c expression in mouse AT and blood.
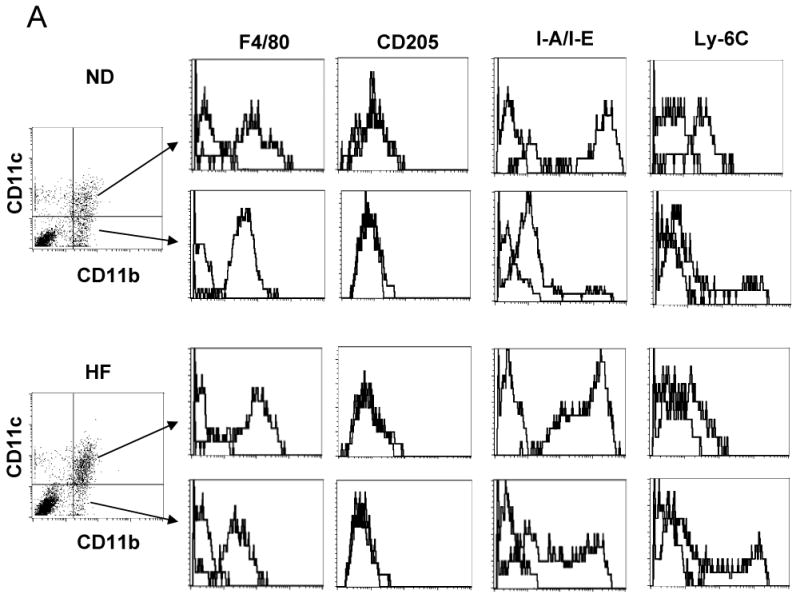
A: Representative FACS analysis showing expression of F4/80, CD205, MHC class II (I-A/I-E), and Ly-6C on CD11b+/CD11c+ and CD11b+/CD11c− cells from AT of lean (ND) or obese (HF) male mice. B: CD11b+/CD11c+ cell numbers in AT of obese (n=9) and lean (n=7) male mice. C: CD11c+ and CD11c− monocytes in blood of obese and lean mice, and in obese mice with normal diet–induced weight loss (HF-WL); n=8–10/group.
CD11c+ monocytes in mouse blood and the effect of weight loss
Using CD204 as a marker of mouse monocytes, and based on CD11c expression, we categorized mouse blood monocytes as CD11c+ or CD11c− monocytes.12 Compared to lean mice, obese mice had an increase in the percentage of CD11c+ monocytes in total leukocytes (Figure 1C). The proportion of CD11c+ monocytes was highly positively correlated with HOMA-IR in mice (r=0.73, P<0.01). Obese mice also had higher CD11c− monocytes in blood than leans (Figure 1C).
Weight loss was induced in obese mice by switching from HFD to ND. By the end of 4 weeks of ND, obese mice had weight loss of 23.5±0.02% of initial body weight and exhibited decreases in both CD11c+ and CD11c− blood monocytes (Figure 1C). Serum MCP-1 level was also higher in obese mice than leans and decreased after weight loss (online Figure IC; please see www.ahajournals.org). An elevation of serum MCP-1 level in lean mice by intravenous injection of MCP-1 increased the proportion of CD11c+ monocytes in total leukocytes (online Figure II; please see www.ahajournals.org).
CD11c, CD11b, and MCP-1 mRNA in human AT
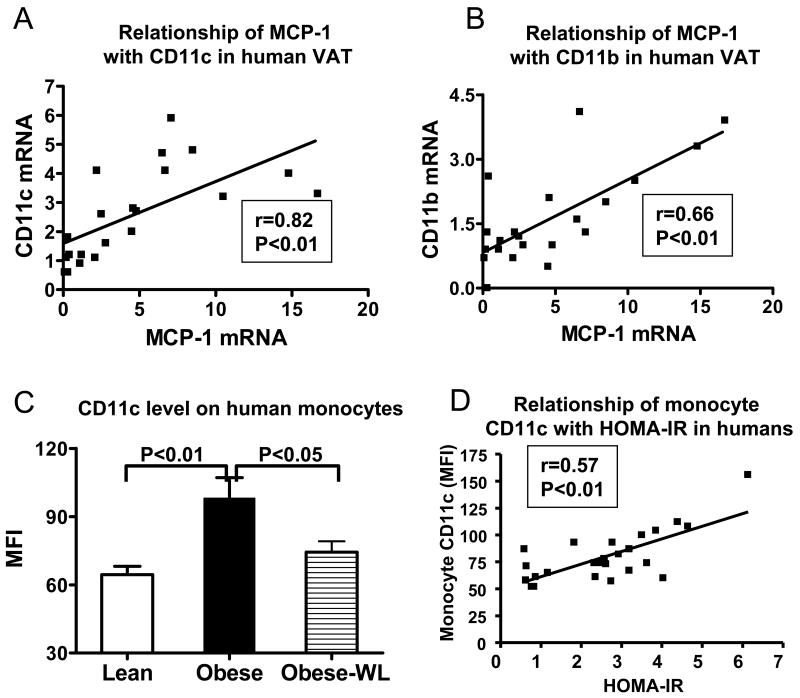
Based on the increased MCP-1 level in AT with obesity and the difficulty in obtaining AT from lean “healthy” human subjects, we examined the relationship between mRNA levels of MCP-1 with CD11c or CD11b in VAT from morbidly obese subjects (age 38.3±1.8 years, BMI 49.9±1.7 kg/m2, n=21), and found that MCP-1 was highly positively correlated with CD11c (Figure 2A) and also positively correlated with CD11b (Figure 2B).
Figure 2. CD11c, CD11b, and MCP-1 in human AT or blood.
A: Correlation of MCP-1 mRNA with CD11c in human VAT as detected by quantitative RT-PCR; n=21. B: Correlation of MCP-1 mRNA with CD11b in human VAT as detected by quantitative RT-PCR; n=21. C: CD11c levels on blood monocytes of obese humans with metabolic syndrome and lean controls, and of obese humans with metabolic syndrome after weight loss (obese-WL) (n=9 for each of obese and lean groups), indicated as mean fluorescence intensity (MFI) by flow cytometry. D: Correlation of monocyte CD11c with HOMA-IR in human subjects; n=26.
CD11c on human blood monocytes and the effect of weight loss
CD11c levels on human monocytes expressed as geometric mean fluorescence intensity (MFI) was examined by flow cytometry. Obese humans with MS had higher CD11c levels on monocytes than did lean healthy controls (Figure 2C), and showed a reduction in monocyte CD11c after weight loss (Figure 2C; weight loss: 11.3±1.1 kg, 9.2±0.5% of initial body weight, online Table I; please see www.ahajournals.org). Monocyte CD11c levels were positively correlated with HOMA-IR in human subjects (Figure 2D).
Effect of CD11c deficiency on mouse weight gain
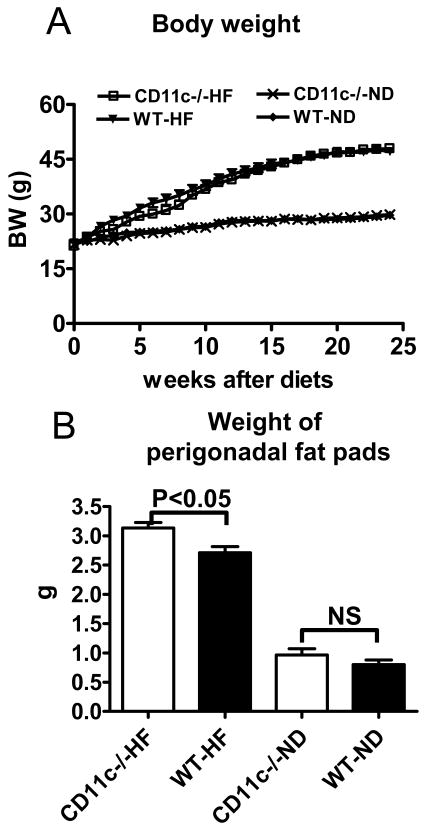
Based on the increased CD11c in obesity, we next examined the effect of CD11c deficiency in mice with diet-induced obesity. When maintained on ND, CD11c−/− mice had body weight comparable to that of WT (Figure 3A). When fed HFD, CD11c−/− and WT mice showed similar weight gain, and had comparable body weight at 24 weeks after HFD (Figure 3A). However, perigonadal fat pads were heavier in CD11c−/− than WT on HFD (Figure 3B).
Figure 3. Body weight and weight of fat pads of CD11c−/− and WT mice.
A: CD11c−/− and WT male mice were fed high-fat diet (HFD) or normal diet (ND) ad libitum, and body weight was monitored weekly for 24 weeks. B: Weights of perigonadal fat pads of CD11c−/− and WT male mice on HFD or ND for 24 weeks. n=12–16/group, NS: not significant.
Effect of CD11c deficiency on leukocyte accumulation in mouse AT
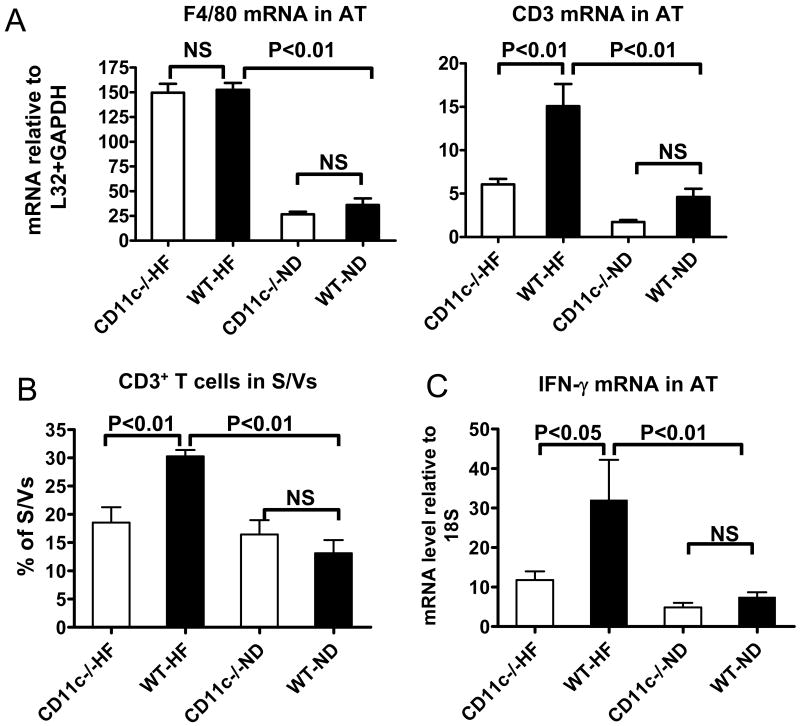
Because monocyte CD11c contributes to monocyte migration and macrophage accumulation in atherosclerotic lesions,12 we determined whether CD11c was involved in leukocyte accumulation in AT by examining leukocytes in perigonadal AT of CD11c−/− and WT mice. Obese WT mice had increased mRNA levels of CD3, a total T cell marker, and F4/80, a commonly used marker for total macrophages,4,5 in AT compared to lean WT. Unexpectedly, obese CD11c−/− mice showed normal levels of F4/80, but decreased levels of CD3, in AT compared to obese WT (Figure 4A). FACS analysis confirmed the lower proportion of T cells in AT S/Vs of obese CD11c−/− mice than obese WT (Figure 4B). The increased T cells in AT of obese WT as compared to lean WT were due to an increase in αβT cells, but not γδT cells (online Figure IIIA; please see www.ahajournals.org). Deficiency of CD11c in obese mice significantly attenuated the increase in αβT cells, but did not alter γδT cells (online Figure IIIA; please see www.ahajournals.org) in AT. Compared to obese WT, obese CD11c−/− mice showed decreases in both CD4 and CD8 mRNA in AT (online Figure IIIB; please see www.ahajournals.org). mRNA level of IFN-γ, a Th1 cytokine that was increased in AT of obese WT compared with lean WT, was also lower in AT of obese CD11c−/− than in obese WT (Figure 4C). However, lean CD11c−/− mice did not show these differences compared with lean WT (Figure 4A–4C, online Figure III; please see www.ahajournals.org). These data indicate that deficiency of CD11c in obese mice reduced T-cell (αβT-cell) number and decreased Th1 response in AT.
Figure 4. T cells in AT of CD11c−/− and WT mice.
A: mRNA levels of CD3 and F4/80 in AT of CD11c−/− and WT mice examined by RPA; n=10/group. B: Total T cells in AT of CD11c−/− and WT mice examined by FACS analysis; n=8 each for CD11c−/− and WT mice on HFD, and n=5 each for CD11c−/− and WT mice on ND. C: IFN-γ mRNA in AT of CD11c−/− and WT mice on HFD or ND as examined by quantitative RT-PCR; n=12–15/group. NS: not significant.
Effect of CD11c deficiency on macrophage activation markers and chemokines in mouse AT
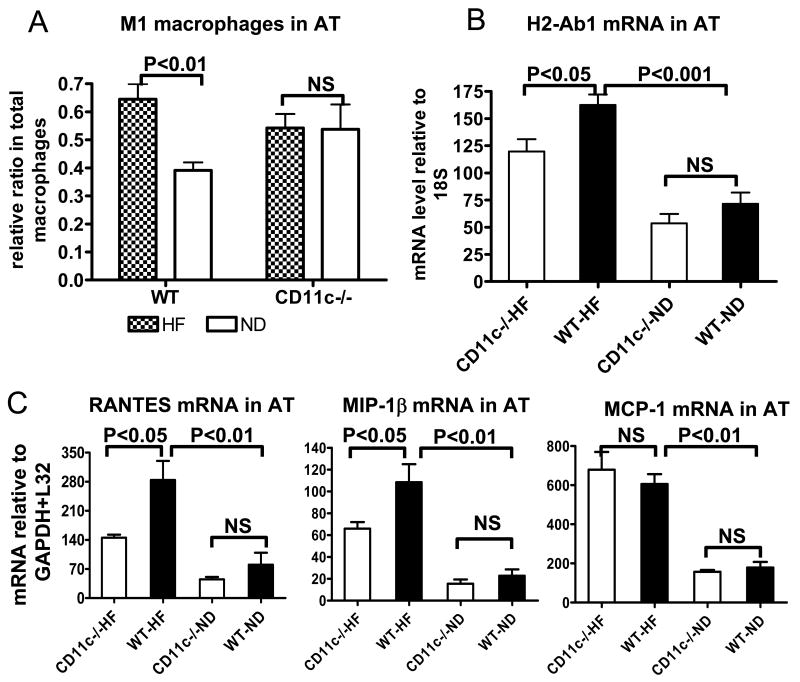
FACS analysis indicated that CD11c was not expressed on T cells from blood or AT of obese or lean WT mice (data not shown), implying that the reduced T cells in AT of obese CD11c−/− mice was not due to CD11c deficiency on T cells. Thus, we examined the effect of CD11c deficiency on macrophage subsets and activation markers in AT. Using CD11b and macrophage galactose N-acetyl-galactosamine specific lectin 1 (MGL1; an alternatively activated macrophage [M2] marker),9 we examined M1 and M2 macrophages in AT by FACS. As expected,9 obese WT mice had increased M1, defined as CD11b+/MGL1− cells, in AT compared to lean WT (Figure 5A). However, obese CD11c−/− mice did not show an increase in M1 in AT compared to lean counterparts (Figure 5A). mRNA level of MHC class II (H2-Ab1) was also examined in AT. Although obese WT and obese CD11c−/− mice had increased MHC class II level compared to their lean counterparts, obese CD11c−/− mice showed lower MHC class II level than obese WT (Figure 5B). mRNA levels of other macrophage activation markers, including arginase I and IL-10 for M2 (online Figure IV; please see www.ahajournals.org) and iNOS for M1 (data not shown), and adiponectin (online Figure IV; please see www.ahajournals.org) were not significantly different between CD11c−/− and WT mice on either HFD or ND.
Figure 5. Macrophages, MHC class II, and chemokines in AT of CD11c−/− and WT mice.
A: Relative ratios of M1 in total macrophages in AT of CD11c−/− and WT mice examined by FACS; n=5–7/group. B: mRNA levels of MHC class II (H2-Ab1) in AT of CD11c−/− and WT mice examined by quantitative RT-PCR; n=12–16/group. C: mRNA levels of RANTES, MIP-1β, and MCP-1 in AT of CD11c−/− and WT mice examined by RPA; n=5-6/group. NS: not significant.
Because increased chemokines such as MCP-1, RANTES, and MIP-1β in AT of obese WT mice may contribute to the increased T cell and/or macrophage accumulation in AT,2,4,5 chemokine levels were compared in AT of obese CD11c−/− and WT mice. Along with lower T-cell numbers, RANTES and MIP-1β mRNA, but not MCP-1 mRNA, was lower in AT of obese CD11c−/− mice than in obese WT mice (Figure 5C). RANTES protein level was also lower in AT of obese CD11c−/− than in obese WT (online Figure V; please see www.ahajournals.org).
Effect of CD11c deficiency on metabolic parameters in obese mice
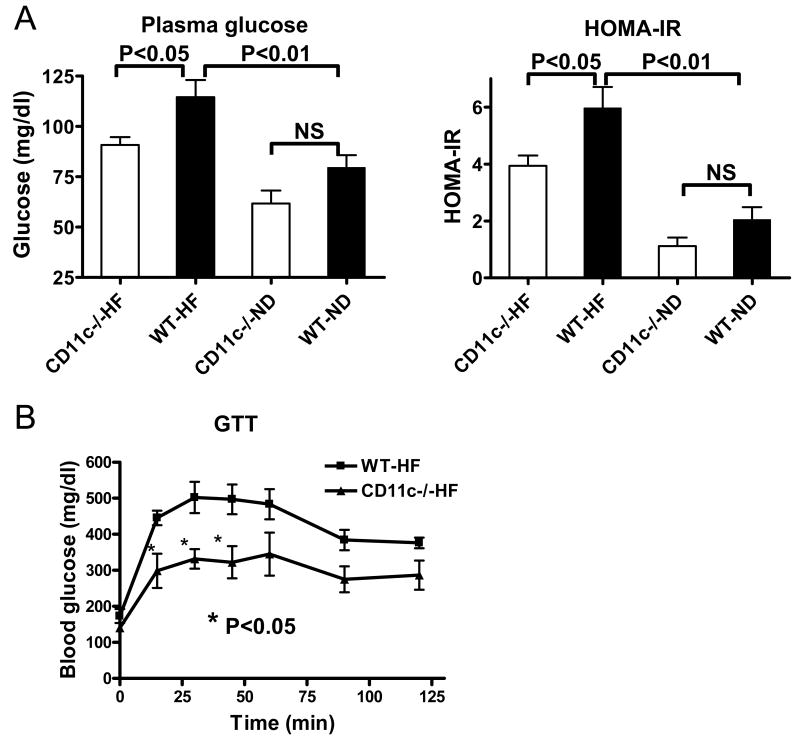
Because AT inflammation, including T cell–related inflammation, may contribute to metabolic abnormalities in obese mice,4,6,7 we examined metabolic parameters in CD11c−/− and WT mice. Fasting plasma insulin and glucose levels and HOMA-IR (Figure 6A) were higher in obese WT than lean WT.2 Compared to obese WT, obese CD11c−/− mice had lower fasting plasma glucose level and HOMA-IR (Figure 6A), and also had ameliorated glucose intolerance as examined by GTT (Figure 6B), suggesting that deficiency of CD11c ameliorated insulin resistance and improved glucose metabolism in obese mice.
Figure 6. Metabolic parameters of CD11c−/− and WT mice.
A: Fasting plasma levels of glucose and HOMA-IR in CD11c−/− and WT mice; n=9–15/group. B: Glucose tolerance test (GTT) in CD11c−/− and WT mice; n=4/group. NS: not significant.
Discussion
In this study, we confirmed that a CD11b+/CD11c+ cell population, which was F4/80+ and Ly-6Clow, was increased in AT of obese mice. We also report here our novel finding that in human VAT, CD11c mRNA was positively correlated with MCP-1. In addition, we made the novel observation that CD11c on blood monocytes was increased in obese mice and obese humans with MS compared to lean controls and was reduced in obese subjects after diet-induced weight loss. Monocyte CD11c was correlated with insulin resistance as measured by HOMA-IR in both mice and humans. More importantly, we report for the first time that deficiency of CD11c in mice decreased AT inflammation and ameliorated insulin resistance and glucose intolerance induced by HFD.
The phenotypic characteristics of CD11b+/CD11c+ cells in mouse AT, with high expression of F4/80, high level of MHC class II, and low level of Ly-6C, indicate that they seem to be “DC-like cells.”6,8,9 Although most of the previous studies designated CD11c+ cells in mouse AT “macrophages” based on the expression of CD11b and F4/80,8-10,16 further study is needed to clarify the functional characteristics of this CD11c+ cell population to determine whether they are DCs or macrophages. Bassaganya-Riera et al categorized AT macrophages into F4/80high and F4/80low macrophages. F4/80high macrophages, which accumulated in AT with obesity, expressed greater amounts of CD11c, MHC II, CD49b, and CX3CR1 than F4/80low macrophages that predominated in AT of lean mice and also increased in obesity.16 These investigators also noted a Ly-6Chigh cell population in F4/80low macrophages,16 which was consistent with our finding of a small population of CD11b+/CD11c− cells that were Ly-6Chigh. Human adiposity is associated with accumulation of CD16− macrophages in AT.17 The origins of AT macrophages/DCs are not clear and need further investigation. Lumeng et al proposed that CD11c+ leukocytes in mouse AT derived from Ly-6Chigh/CD11c− monocytes based on the “proinflammatory” nature of Ly-6Chigh monocytes18,19 and a reduction of AT CD11c+ leukocytes in CCR2−/− mice.9 However, our current study and that of Bassaganya-Riera et al16 suggested that CD11c+ leukocytes in mouse AT resembled blood Ly-6Clow/CD11c+/CX3CR1high monocytes, a finding consistent with that on CD11c+ cells in atherosclerotic lesions.20,21 Although CD16− macrophages in human AT may originate from blood CD16− monocytes, these macrophages lacked CCR2 and CD62L expression.17 We do not exclude a potentially important role of Ly-6Chigh monocytes. However, based on the observations made by us and others,16 we propose an important role of CD11c+/Ly-6Clow monocytes in AT inflammation. As additional support, CD11c+ monocytes were increased in blood of obese mice as discussed below. The “patrolling” Ly-6Clow monocytes have been shown to be responsible for an early inflammatory response22 and to be more prone to developing into CD11c+ cells in atherosclerotic lesions.20
Both CD11c+ and CD11c− monocytes were increased in blood of obese mice. Blood CD11c+ monocytes appear to be mature monocytes, which derive from CD11c− monocytes, and blood monocytes undergo rapid turnover.20 One potential explanation for the increases in both CD11c+ and CD11c− monocytes in blood of obese mice is that increased stimuli such as MCP-1 in blood enhance differentiation of CD11c− to CD11c+ monocytes, which increases CD11c+ monocytes and also accelerates monocyte (CD11c−) release from bone marrow, thereby increasing CD11c− monocytes as well. Indeed, short-term administration of MCP-1 increased CD11c+ (online Figure II; please see www.ahajournals.org) or Ly-6Clow monocytes23 in blood of lean mice. Deficiency of MCP-1 or its receptor has been shown to reduce both Ly-6Chigh and Ly-6Clow monocytes in mouse blood23,24 because of impaired monocyte egress from bone marrow.24 The functional consequence of increased CD11c on blood monocytes in obesity may include a contribution to AT inflammation as discussed below, and also to the high risk for atherosclerotic CVD in obese individuals based on an important role of CD11c in atherogenesis.12
Patsouris et al recently showed that CD11c+ cells contributed to obesity-linked AT inflammation and insulin resistance.11 Our current study provides the first evidence for an essential role of CD11c in AT inflammation and insulin resistance induced by HFD. Based on the role of CD11c in monocyte adhesion and migration,12 we had expected that deficiency of CD11c would reduce macrophage accumulation in AT with obesity. However, compared to obese WT, obese CD11c−/− mice did not show a difference in the level of F4/80, a commonly used total macrophage marker, but exhibited decreased T-cell accumulation and activation (IFN-γ) in AT, indicating that CD11c is essential for T-cell accumulation and activation in AT with obesity. Patsouris et al found that ablation of CD11c+ cells in obese mice decreased plasma levels of IFN-γ.11 Another report indicated that CD11c deficiency reduced T-cell infiltration in spinal cord in an experimental autoimmune encephalomyelitis model.25 Research from Leo Lefrançois's group at the University of Connecticut showed that CD11c plays a critical role in T-cell activation (unpublished data, 2009). Thus our data and those of others unveil an important link between CD11c and T-cell behaviors. The mechanisms for this link remain to be defined. CD11c+ leukocytes from AT of obese WT mice displayed characteristics of M1.9,10 We postulate that the failure of macrophages in AT of obese CD11c−/− mice to polarize to M1, with reduced secretion of chemokines (such as RANTES), may contribute to the decreased T-cell accumulation and activation in AT. In addition, AT CD11c+ cells expressed high levels of MHC class II. CD11c deficiency in obese mice decreased MHC class II expression in AT, which may impair antigen-presenting cell–T cell interaction, and thus could also contribute to reduced T-cell proliferation and activation in AT.
T cells, with increased accumulation and activation in AT with obesity,2,26 have recently been shown to contribute to metabolic dysfunctions in diet-induced obesity.6,7 Consistent with these studies, the attenuated T-cell accumulation and activation in AT of obese CD11c−/− mice was accompanied by improved insulin resistance and glucose intolerance, suggesting that CD11c also plays a role in metabolic abnormalities in diet-induced obesity.
In summary, we report that CD11c expression is increased on AT leukocytes and blood monocytes with diet-induced obesity. The increased expression of CD11c may enhance accumulation and/or activation of macrophages and/or DCs in both AT and the arterial wall, and may contribute to T-cell accumulation and activation, thereby accelerating AT inflammation and atherogenesis. The important role of CD11c in AT inflammation and in insulin resistance and glucose intolerance with diet-induced obesity, as demonstrated in mice deficient in CD11c, suggests that CD11c may be a novel therapeutic target for obesity-related diseases.
Supplementary Material
Acknowledgments
a) Sources of Funding — This work was supported by grants: R01DK078847 (to C.M.B.) from the National Institutes of Health; 6250-51000-046 (to C.W.S.) from the U.S. Department of Agriculture; and from the American Heart Association (to H.W.), the American Diabetes Association, and The Methodist Research Hospital Foundation (to C.M.B.).
b) Acknowledgments — We thank Kerrie Jara for editorial assistance.
Footnotes
c) Disclosure — The authors have no conflicts of interest to disclose.
References
- 1.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, Ghosh S, Perrard XYD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 7.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol. 2006;291:C1232–1239. doi: 10.1152/ajpcell.00008.2006. [DOI] [PubMed] [Google Scholar]
- 9.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 11.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Gower RM, Wang H, Perrard XYD, Ma RD, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–146. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumie A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 19.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 23.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 24.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullard DC, Hu X, Adams JE, Schoeb TR, Barnum SR. p150/95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. Am J Pathol. 2007;170:2001–2008. doi: 10.2353/ajpath.2007.061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-γ, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.