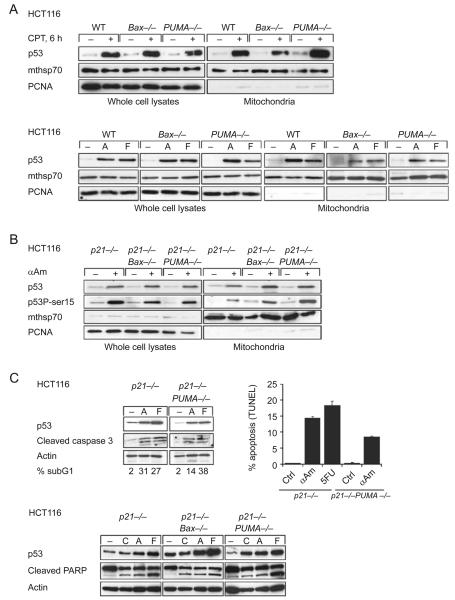
Figure 1.
Death stimulus-induced translocation of p53 to mitochondria is independent of Puma and Bax. (A) HCT116 cells of the indicated genotypes were treated with camptothecin (CPT), α-amanitin (A) or 5′ fluorouracil (F), or left untreated. Whole cell lysates and purified mitochondria (equal total protein) were immunoblotted. Stress-induced p53 translocation to mitochondria is completely unaffected by Puma and Bax deficiency. Mthsp70 is a mitochondrial and PCNA a nuclear contamination marker. (B) p21−/− HCT116 cells or Bax- and Puma-deficient isogenic derivatives were treated with transcriptional inhibitor α-amanitin and characterized as in (A). (C) Blockade of the transcriptional arm of the p53 response does not significantly interfere with caspase 3 activation/PARP cleavage and apoptosis in HCT116 cells. Also, baseline levels of cytosolic Puma promote the mitochondrial arm of p53 apoptosis (see text). Cells of the indicated genotypes were treated with camptothecin (CPT, 5 μM), α-amanitin (A or αAM, 10 μg/ml) or 5′ fluorouracil (F or 5FU, 375 μM) for 24 h (upper panels) or 48 h (lower panels) and immunoblotted as in (A). Apoptosis was assessed by sub-G1 fraction and TUNEL.