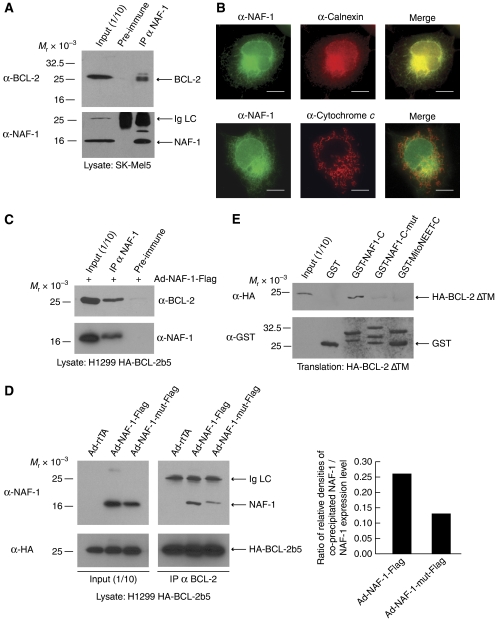
Figure 2.
The NAF-1/BCL-2 interaction. (A) Co-immunoprecipitation of endogenous NAF-1 and BCL-2. Lysates from SK-Mel5 cells were collected and immunoprecipitation was performed with anti-NAF-1 antibody. The precipitate was subjected to immunoblotting with anti-BCL-2 and anti-NAF-1. (B) H1299 cells were fixed and double stained with anti-NAF-1 and anti-Calnexin or anti-Cytochrome c antibodies. Scale bar represents 10 μm. (C) Co-immunoprecipitation of NAF-1-Flag and HA-BCL-2b5. Lysates from H1299 HA-BCL-2b5 cells infected with Ad-NAF-1-Flag were collected and treated as in (A). (D) Mutations in the CDGSH iron-binding domain of NAF-1 interfere with NAF-1 binding to BCL-2. H1299 HA-BCL-2b5 cells were infected with either Ad-rtTA, Ad-NAF-1-Flag, or Ad-NAF-1-mut-Flag (C99S C101S C110S H114Q). Lysates were treated as in (A). Densitometric analysis was performed using Scion Image software to quantify expression and co-precipitated levels of NAF-1-Flag and NAF-1-mut-Flag. Graph depicts the ratio of co-precipitated protein to expression level. (E) A functional CDGSH iron-binding domain is necessary, but not sufficient for the interaction between the cytosolic domains of NAF-1 and BCL-2. HA-BCL-2 ΔTM was in vitro translated in rabbit reticulocyte lysate and equivalent aliquots were added to each GST pull-down reaction. GST-fusion proteins used were GST alone, GST-NAF1-C, GST-NAF1-C-mut (C99S C101S C110S H114Q), and GST-MitoNEET-C. The proteins were detected using anti-HA and anti-GST.