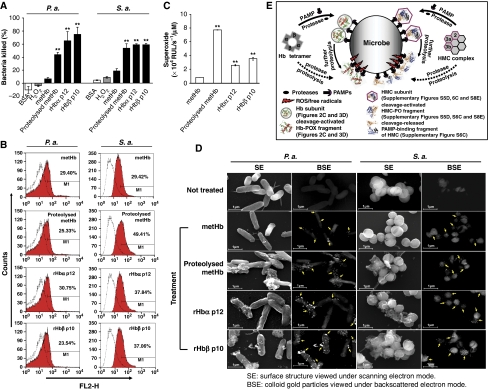
Figure 5.
The biological effects of the activated antimicrobial centres in Hb, and a model for the ‘structure-function reprogramming' of respiratory proteins. (A) The bactericidal activities of Hb fragments were determined by bacterial viable count. The % killing was calculated based on the negative controls. (B) The binding of Hb fragments to the bacteria was analysed by flow cytometry. The fluorescence shift (red) was plotted against the negative control (white) bacteria incubated without the protein. FL2-H denotes the relative fluorescence intensity. (C) The POX cycle activity, represented by the production of superoxide, of 1 μM of the Hb fragments measured by CLA-CL assay. (D) SEM analysis of the bacteria treated with immunogold-labelled Hb fragments. The SE and BSE modes were used to view the bacterial surface morphology and the colloidal gold particles, respectively. The colloidal gold (arrows) shows the specific binding of Hb fragments to the bacteria. Magnification is × 25 600. **Statistical significance compared to that of the metHb, P<0.05, was calculated by Student's t-test. S.a.: S. aureus and P.a.: P. aeruginosa. (E) During infection, the extracellular microbial proteases cause limited proteolysis of the Hb/HMC complexes. The superficial subunits/regions of Hb/HMC can be directly proteolysed ( ), or alternatively, the released subunits bind to the bacterial surface (
), or alternatively, the released subunits bind to the bacterial surface ( ) through PAMPs and undergo further proteolysis, both of which expose ‘dual-action antimicrobial centres' (PAMP-binding and free radical production by POX cycle or PO) in the fragments. Co-localization of these two activities in the same fragments elicits concurrent ‘anchor-and-attack' strategy to kill the invading microbe and effectively stall the initial phase of infection.
) through PAMPs and undergo further proteolysis, both of which expose ‘dual-action antimicrobial centres' (PAMP-binding and free radical production by POX cycle or PO) in the fragments. Co-localization of these two activities in the same fragments elicits concurrent ‘anchor-and-attack' strategy to kill the invading microbe and effectively stall the initial phase of infection.