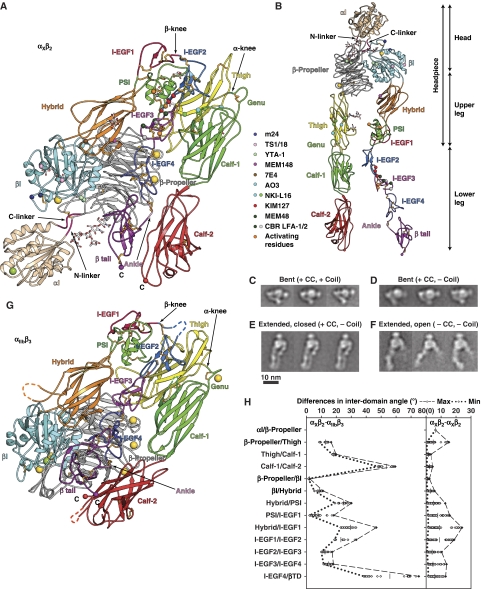
Figure 2.
Structure of CR4, integrin αXβ2. (A) Cartoon of αXβ2 molecule 1 in lattice A and (B) an extended model made by adjusting domain interfaces at and near the knees. Disulfides are gold sticks, and glycans are sticks with white carbons. Ca and Mg ions are gold and green spheres, respectively. Smaller spheres show Cα atoms of conformation-associated epitopes and C-terminal residues. (C–F) Representative αXβ2 EM class averages. The presence (+) or absence (−) of a disulfide after the αX and β2 C-termini (CC) and subsequent linker and coiled-coil (coil) is indicated. (G) Cartoon of αIIbβ3 (Zhu et al, 2008) in same orientation and style as αXβ2 in (A). (H) Differences in interdomain angles between 10 molecules of αXβ2 and 2 molecules of αIIbβ3 (left panel), or among 10 molecules of αXβ2 (right panel). The mean values of interdomain angles are shown as bars and the maximal and minimal angles are shown in dashed or dotted lines, respectively.