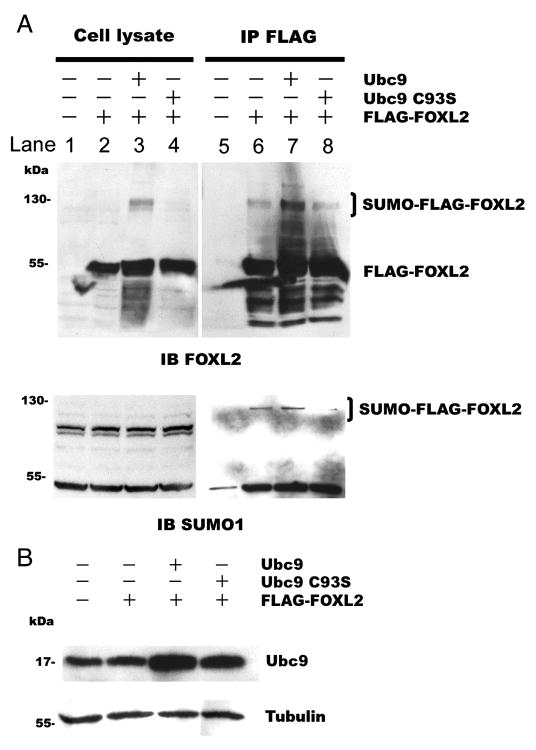
Fig. 4.
Overexpression of Ubc9 enhances the sumoylation of FOXL2. CHO cells were transfected with 0.4μg FLAG-FOXL2 plasmid alone, 0.4μg FLAG-FOXL2 and 2μg Ubc9 plasmids, or 0.4μg FLAG-FOXL2 and 2μg dominant negative mutant Ubc9 C93S plasmids. The cell lysates were immunoprecipitated. Proteins in the cell lysates and immunoprecipitates were resolved by SDS-PAGE and immunoblotted with an antibody to FOXL2 or SUMO1 (A) or Ubc9 (B). A. Cells transfected with FOXL2 along or with wild type or mutant Ubc9 exhibit a 60 kDa FLAG-FOXL2 product (cell lysate, lane 2, 3 & 4). After immunoprecipitation of FLAG-tagged proteins, cells transfected with FLAG-FOXL2 alone exhibit both a 60 kDa FLAG-FOXL2 product and a 120 kDa sumoylated FLAG-FOXL2 product (SUMO-FLAG-FOXL2) (lane 6). Co-expression of FLAG-FOXL2 with Ubc9 increased the amount of the 120 kDa product (lane 7), but co-expression with dominant negative mutant Ubc9 C93S did not (lane 8). B. Transfection with wild type Ubc9 or dominant negative mutant Ubc9 C93S increased the amount of Ubc9 in these cells. The blots of the cell lysates were then re-probed with a tubulin antibody to control for equal loading. The experiment was repeated three times with similar results.