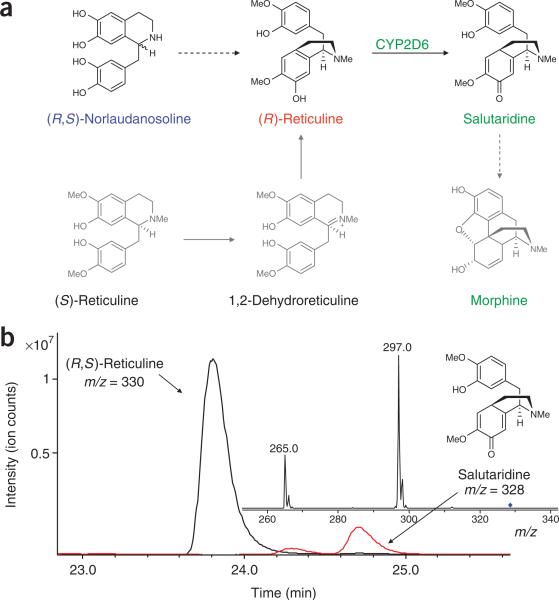
Figure 5.
Microbial production of morphinan alkaloids. (a) Native and engineered pathways for the synthesis of morphinan metabolites. Color schemes and notation follow that defined in Figure 4. In the engineered pathway, (R)-reticuline is produced from (R)-norlaudanosoline. A heterologous human cytochrome P450 (CYP2D6) enzyme can convert (R)-reticuline to the first morphinan alkaloid salutaridine. In the native plant pathway, only (S)-reticuline is produced, and two additional enzymes are required to produce (R)-reticuline, which is used in the synthesis of the morphinan alkaloids. (b) LC-MS/MS analysis of the growth medium of engineered yeast strains supplemented with 4 mM norlaudanosoline and grown for 48 h confirms salutaridine production. Extracted ion chromatograms are shown for reticuline (black) and salutaridine (red) produced by CSY489 and are representative of analogous strains. The salutaridine 328 ion elutes at the same time as (S)-scoulerine, but exhibits a distinctly different fragmentation pattern. The major ions (m/z = 297 and 265) are consistent with the expected fragments reported in the literature for salutaridine44. Strains lacking the CYP2D6 coding sequence did not produce the metabolite peak identified as salutaridine.