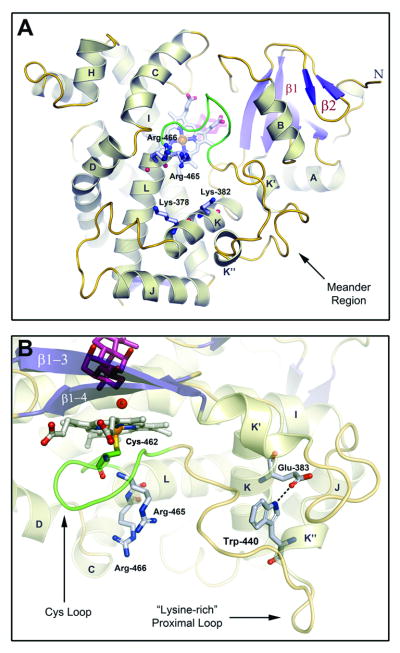
Fig 5. Adaptations for Adrenodoxin Binding.
A) CYP24A1's proximal surface is shown below the heme prosthetic group with key structural elements implicated in adrenodoxin binding noted. Basic residues from helices B, C, D, J, K and L, the Cys-Loop and the (bacterial) meander region line the positively-charged Adx binding. Fully-conserved residues from helices K (K378,K382) and L (R465,R466) known to mediate adrenodoxin binding and electron transfer in related P450s are labeled [52-55]. B) A conserved tryptophan residue (W440), from the K″ helix of mitochondrial P450s, forms a salt-bridge to the K-helix via a fully-conserved glutamate residue (E383) that may contribute to the display of the meander's lysine-rich, proximal loop, that is associated with Adx binding [57]. Residues from the L-helix (R465,R466) and Cys-loop (M462), implicated in the electron shuttle process are shown below the heme in close proximity to the K-helix. The lower portion of the active-site is also shown with CHAPS (pink) positioned above the water (WAT6) bound heme iron.