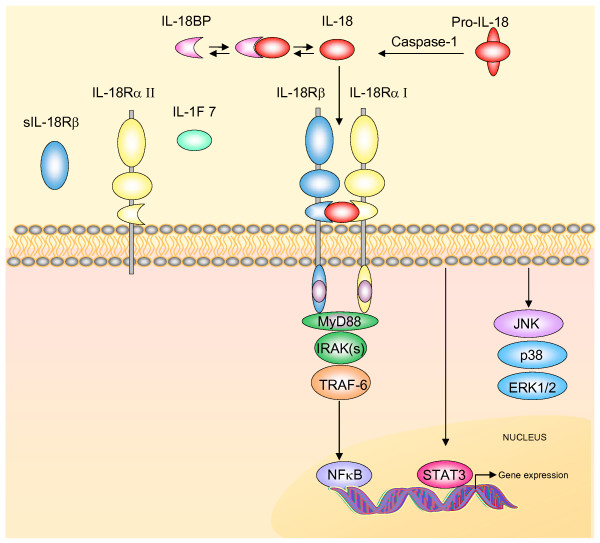
Figure 1.
The IL-18 system. Active IL-18 is produced and secreted after proteolitic cleavage of the biological inactive precursor Pro-IL-18 by caspase-1. IL-18 action can be regulated by the IL-18 binding protein (IL18-BP) that binds IL-18 with high affinity and inhibits its function. Free IL-18 binds to a specific heterodimeric cell surface receptor, a member of the IL-1 receptor/Toll like receptor superfamily comprised of two subunits, IL-18Rα (here referred to as IL-18RαI) and IL-18Rβ, both with three extracellular Ig-like domains and one intracellular portion containing the Toll/IL-1R domain (TIR). Interaction of IL-18 with the IL-18Rα stabilizes its interaction with IL-18Rβ and with the adaptor protein MyD88 via the TIR domain. This initiates signal transduction by recruitment of the IL-1 receptor activating kinase (IRAK). IRAK autophosphorylates and dissociates from the receptor complex subsequently interacting with the TNFR-associated factor-6 (TRAF6) eventually leading to nuclear translocation of the nuclear factor κB (NF-κB). Engagement of the IL-18R complex can also activate STAT3 and the mitogen-activated protein kinase (MAPK) p38, JNK and ERK. One truncated variant of IL-18Rα (IL-18RαII) lacking the intracellular TIR domain, and one soluble isoform of the IL-18Rβ (sIL-18Rβ) were demonstrated in vivo in the mouse brain and in the rat and human brain, respectively. These isoforms originating from differential splicing are proposed to be decoy receptors and possible negative regulators of IL-18 fuction. IL-1F7 is another proposed regulator of IL-18 action (see text for details).