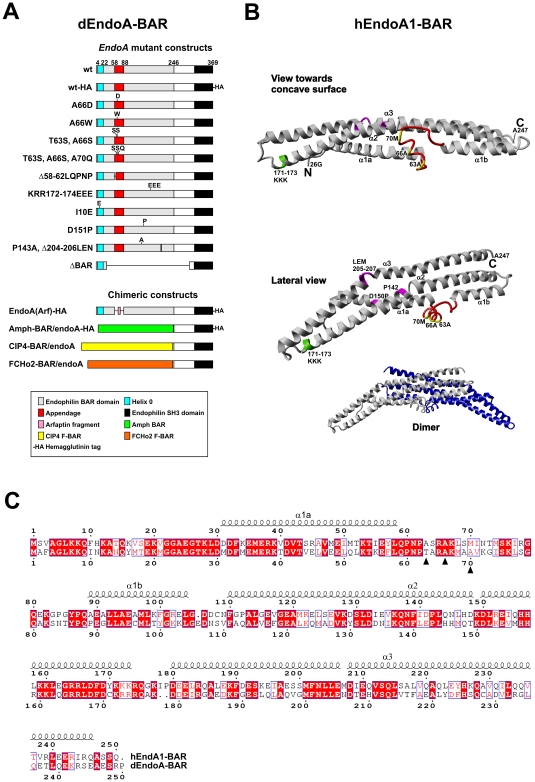
Figure 1. Targeted mutations in dEndoA-BAR and their relationship to the structure of hEndoA1-BAR.
A, Schematic representation of the mutations introduced in the rescue constructs encoding dEndoA-BAR. B, Mutations homologous to the mutations in dEndoA-BAR (A), mapped onto the tertiary structure of hEndoA1-BAR monomer [PDB code 1X03A, 8]. The central helix-loop appendage (red) and the residues constituting the hydrophobic ridge (yellow) are indicated. The residues mutated to change the BAR domain curvature are also indicated (pink), as are the three electropositive lysine residues that were mutated to electronegative glutamic acid residues (light green). The inset at the lower right shows the BAR dimer, with the two monomers colored gray and blue. C, Primary structure alignment of hEndoA1-BAR (accession BAE44459.1; top) and dEndoA-BAR (accession CAD24682.1; bottom). The alpha-helical secondary structure is indicated by squiggles, based on the hEndoA1-BAR structure. The residues associated with the hydrophobic ridge are also indicated (closed triangles).