Abstract
Increased attention to timely diagnosis motivated us to study 5483 patients diagnosed with multiple myeloma using Medicare claims linked to tumor registries in the Surveillance, Epidemiology and End Results programme. We calculated the time between initial visits for anemia or back pain and for myeloma diagnosis, and used logistic regression to predict the likelihood of diagnostic delay, and also the likelihood of renal or skeletal complications. The median time between sign or symptom and myeloma diagnosis was 99 days. Patients with anemia, back pain and comorbidities were more likely to experience diagnostic delay (OR 1.6, 95% CI 1.3–2.0). Diagnosis while hospitalised (OR 2.5, 95% CI 2.2–2.9) and chemotherapy treatment within 6 months of diagnosis (OR 1.4, 95% CI 1.2–1.6) significantly predicted complications; diagnostic delay did not (OR 0.9, 95% CI 0.8–1.1). Our data suggest that complications are more strongly associated with health status and myeloma severity than with diagnostic delays.
Keywords: Multiple myeloma, diagnostic delay, health services research, quality of care
Introduction
Stakeholders in both the United States [1] and the United Kingdom [2] have identified timeliness of patient care as a high priority for quality improvement efforts. One aspect of timeliness of care is the interval between recognition of a sign or symptom and a definitive diagnosis by a health care provider. Rapid diagnoses may result in favorable patient outcomes, including fewer complications and reduced mortality, as well as greater satisfaction with care and a better perceived quality of life [3]. Concerns over delayed referral and diagnosis are especially relevant for patients with cancer because of an increased likelihood of disease-free survival when tumors are detected at earlier stages [4-11]. In the year 2000, the United Kingdom Department of Health launched the Cancer Referral Guidelines, which set a 2-week target for patients with suspected cancers to be seen by an appropriate consultant [12]. To date, mixed results from this initiative have been reported [13-16].
Barriers to timely cancer detection may be categorised as those related to practitioners, patients and health care systems [17]. Health service researchers have studied predictors and adverse outcomes associated with delays in diagnosis and treatment of cancer, but the majority of these studies have focussed on solid tumors, notably breast cancer [18-22].
Analysis of results from the ‘1999–2000 National Survey of NHS Patients – Cancer’ reveals that delays in diagnoses vary for six tumor types studied: while gender, age, socioeconomic status and race/ethnicityare all significant predictors of delay, the effects of these factors are not uniform across tumors [23-25]. In a review of diagnostic delays reported in multiple countries, we identified no variables consistently associated with delays [26]. Further, we found no empirical studies that examined diagnostic delays in multiple myeloma.
Many patients with myeloma develop renal dysfunction and skeletal complications, such as fracture or spinal cord compression, which decrease quality of life and increase mortality [27]. These adverse outcomes may be related to variations in the time required to obtain myeloma diagnoses. For example, data from 92 patients in a myeloma clinic were analysed for the frequency of relapse and death based on delayed presentation [28]. Forty-four percent of patients with a delay in diagnosis exceeding 6 months had relapsed or died at the end of the study period, compared with 21% in the group of patients with shorter delays (p < 0.05). These delays may occur because of non-specific presenting symptoms. A retrospective review of 1027 cases of myeloma diagnosed between 1985 and 1998 at the Mayo Clinic identified a high prevalence of anemia and bone pain as an initial sign and symptom [29]. These conditions were also observed in a Dutch registry of 127 patients diagnosed with myeloma between 1991 and 1993, 37% of whom did not have myeloma according to the initial differential diagnosis [30].
From these retrospective, single-site studies of patients with myeloma, we cannot clearly identify consistent predictors of delay, nor can we link delays in diagnosis and treatment to the likelihood of complications. Moreover, very few studies address the issue of delay in patients with hematological malignancies. The present report attempts to bridge these gaps by identifying the predictors of diagnostic delay and associated complications in patients with multiple myeloma.
Methods
Data sources
We used the Surveillance, Epidemiology and End Results (SEER) – Medicare data set to identify patients diagnosed with multiple myeloma. Through collaborations among the National Cancer Institute, the Center for Medicare and Medicaid Services and participating state and regional cancer registries, the SEER-Medicare data set combines cancer registry information with complete claims data for adults age 65 and above enrolled in traditional Medicare Parts A and B [31]. The analysis sample contained patients diagnosed with multiple myeloma between 1 February 1992 and 31 December 2002. The 16 registries in this dataset cover a representative sample of 26% of the population of the United States. Prior studies have documented that the participating tumor registries captured ~97% of incident cases reported by hospitals [32]. The data set is particularly rich in racial and ethnic minority populations [33,34]. Our study protocol was granted exempt review from the Dana-Farber Cancer Institute Institutional Review Board. The principal investigator executed a signed data-use agreement with the SEER-Medicare coordinating center.
Study sample
We used the cancer registry data to identify patients with multiple myeloma diagnoses, confirmed via pathologic, radiologic or laboratory findings. Patients with multiple cancers were eligible if multiple myeloma was the first cancer diagnosed. The SEER cancer diagnosis date (henceforth defined as diagnosis date) had to be on, or after, the patient’s date of Medicare enrollment and patients had to survive six or more months following diagnosis. To measure health care utilisation for signs or symptoms, patients had to be continuously enrolled in both Part A and Part B Medicare in the year prior to their diagnosis. Following a definitive diagnosis, patients had to survive for at least 6 months so we could examine use of chemotherapy, incidence of complications and performance of diagnostic studies. We identified 8735 patients in the participating registries who were diagnosed with multiple myeloma between 1992 and 2002 and who met eligibility criteria.
Of these, we excluded 2952 patients based on the following: diagnosis at autopsy, eligible for Medicare because of end-stage renal disease or disability and participation in Medicare health maintenance organisations during the study period. Forty patients with a claim for amyloidosis made prior to their myeloma diagnosis were excluded, and 260 patients were not included because no physician, outpatient or hospital claims specifying a myeloma diagnosis were available. The final analysis sample consisted of 5483 patients. We defined the study period for an individual patient as starting the year prior to diagnosis date, and ending up to 6 months following the diagnosis date.
Study variables
Patient characteristics
The Patient Entitlement and Diagnosis Summary File was used to measure patient characteristics, including age, gender, race/ethnicity, geographic region of residence, and residence in an urban versus rural area. Medicare inpatient (MED-PAR), outpatient (OUTSAF) and physician (NCH) files were used to construct a modified Charlson comorbidity score for all patients by reviewing claims for the year prior to the SEER diagnosis date [35]. We measured all physician visits and hospitalisations for patients by summing the number of claims filed during the year prior to diagnosis.
Signs and symptoms, diagnostic and therapeutic procedures
Based upon our review of the literature [27-30], and in consultation with clinicians treating patients with myeloma, we matched signs and symptoms frequently associated with myeloma – determined to be anemia, packed red blood cell (PRBC) transfusion, and back pain – to diagnoses and procedures from the claims data using International Classification of Diseases, 9th edition (ICD-9) and current procedural terminology (CPT) codes. We created dichotomous variables to identify the presence or absence of claims for these signs and symptoms during the 6 months preceding the SEER diagnosis date. We also considered certain procedures to be proxies for the diagnosis of multiple myeloma, and identified them using CPT and ICD-9 procedure codes. These procedures included urine or serum protein electrophoresis (PEP), bone marrow biopsy and bone scan or skeletal survey. We also identified bisphosphonate or chemotherapy infusions following diagnosis. A table with all of the codes that were used to create these variables is available in Appendix.
Myeloma diagnosis, delay and complications
Because of reporting lags, SEER diagnosis dates have a window of ±30 days for accuracy [36]; all calculations of the time between diagnosis dates and dates of signs, symptoms or diagnostic procedures were thus adjusted for these lags; the final results were unchanged when unadjusted windows were used. We measured the number of days between the first claim of a sign or symptom of myeloma and the diagnosis date (with the above-mentioned adjustment).
Literature review and consultation with myeloma clinicians led us to identify ICD-9 and CPT codes that reflect complications occurring up to 6 months following the diagnosis date; in this analysis we focus on renal failure and skeletal complications (see Appendix). Patients were excluded from the analysis of complications if they had claims for either of these conditions in the year prior to the diagnosis date.
Data analysis
Examination of delays
For the 3831 patients who had a claim for a myeloma-related sign or symptom preceding myeloma diagnosis, we calculated the number of days between the diagnosis date and the initial claim for a sign or symptom. We used an ordered logistic regression model to estimate the likelihood of an increasing gap between the initial sign or symptom date and the diagnosis date based on the quartile distribution of diagnosis times for the sample. Patient demographics, comorbidities, health care utilisation in the prior year and initial source of diagnosis were considered for the model, and variables were retained using the ‘Purposeful Selection’ algorithm [37]. Parameter estimates changed for the presence of a sign or symptom when comorbidity values were entered into the model. Thus, an interaction term was added to reflect the presence of one or more comorbidities, and the presence of both anemia and back pain (prior to diagnosis). The parameter estimates, variance and covariance were used to calculate the effect sizes for patients with at least one comorbidity, plus anemia and back pain, versus patients with one comorbidity and either anemia or back pain [38].
Predictors of complications
After excluding patients with a renal or skeletal complication recorded in claims prior to the diagnosis date, 5406 patients were available for analysis. A logistic regression model predicted the likelihood of a renal or skeletal complication subsequent to the diagnosis date. The main independent variable of interest was delay (defined as exceeding the sample’s median value) between sign or symptom and diagnosis. Additional covariates included the demographic variables previously mentioned, as well as diagnostic or therapeutic procedures performed. Sensitivity analyses were performed to examine the effect of a three-category variable of diagnostic delay (delay, no delay, missing sign or symptom information). We also estimated the likelihood of complication when diagnostic delays exceeded the tenth percentile of the sample distribution. We also estimated a model that includes an interaction of comorbidity and diagnostic delay.
We used SAS 9.1.3 (Cary, NC) for all analyses. Coefficients obtained from logistic regression models were expressed as odds ratios (ORs), with corresponding 95% confidence intervals (CIs).
Results
Patient characteristics
Of the 5483 patients who met study criteria (full sample), 3831 (70%) had a claim for a sign or symptom prior to the diagnosis of multiple myeloma. Table I compares the patient characteristics by three subsamples: (a) patients who had no claim for sign or symptom prior to diagnosis (no claim); (b) patients who had a sign or symptom, but were diagnosed sooner than the median period of time for the sample (no delay), and (c) patients who had a sign or symptom, and were diagnosed later than the median period of time for the sample (delay). Significant differences were observed for age, race, gender, comorbidity, geographic region and prior physician visits. Patients who experienced delay were an average of 1 year older. When comparing the distribution of race by sample, a larger proportion of black patients (14.9%) experienced a diagnostic delay than is observed in the other subsamples (11.1% and 13.0%), and a similar pattern was observed for women (58.0% in the delay sample, 48.3% and 49.1% in the no claims or no delay sample, respectively). Significantly more patients in the delay sample had two or more comorbidities, and had more physician visits in the year prior to diagnosis.
Table I.
Characteristics of study patients, by analytic sample (n = 5483)
| No sign or symptom reported (n = 1652) |
No delay (n = 1913) |
Delay (n = 1918) |
p | |
|---|---|---|---|---|
| Age in years, mean ± STD | 75.9 ± 6.4 | 75.7 ± 6.4 | 76.9 ± 6.6 | <0.0001 |
| Race, n (%) | ||||
| White | 1415 (85.7) | 1588 (83.0) | 1542 (80.4) | |
| Black | 184 (11.1) | 248 (13.0) | 286 (14.9) | |
| Other | 53 (3.2) | 77 (4.0) | 90 (4.7) | <0.01 |
| Gender, n (%) | ||||
| Male | 854 (51.7) | 973 (50.9) | 805 (42.0) | |
| Female | 798 (48.3) | 940 (49.1) | 1113 (58.0) | <0.0001 |
| Urban resident, n (%) | 1508 (91.3) | 1738 (90.1) | 1725 (90.0) | 0.36 |
| Charlson score, n (%) | ||||
| 0 | 687 (41.6) | 845 (44.2) | 679 (35.4) | |
| 1 | 331 (20.0) | 350 (18.3) | 494 (25.8) | |
| 2+ | 147 (8.9) | 190 (9.9) | 368 (19.2) | |
| No claims | 487 (29.5) | 528 (27.6) | 377 (19.7) | <0.0001 |
| Region of residence, n (%) | ||||
| Northeast | 308 (18.6) | 347 (18.1) | 315 (16.4) | |
| South | 180 (10.9) | 230 (12.0) | 216 (11.3) | |
| Midwest | 407 (24.6) | 506 (26.5) | 549 (28.6) | |
| West | 757 (45.8) | 830 (43.4) | 838 (43.7) | 0.10 |
| Physician visits in year prior to myeloma diagnosis, median, IQR |
7, 4-13 | 9, 5-13 | 13, 8-19 | <0.0001 |
Note: Percentages may not total 100 percent due to rounding. STD, standard deviation; IQR, interquartile range.
The frequency of claims for a sign or symptom prior to myeloma diagnosis was high; Figure 1 shows frequencies of claims for anemia or PRBC transfusions (50%), back pain (39%) and for both anemia/transfusion and back pain (19%) prior to myeloma diagnosis. Roughly half of the patients had a claim for PEP of the urine or serum, 37% had a bone marrow biopsy and 42% had either a bone scan or skeletal survey. Because the ICD-9 and CPT codes changed during the study period, we were unable to clearly distinguish between bone scans and skeletal surveys. Less than a quarter of study patients received both PEP and bone marrow biopsy, and only 17% received all three diagnostic tests (PEP, bone marrow biopsy and radiographic evaluation).
Figure 1.
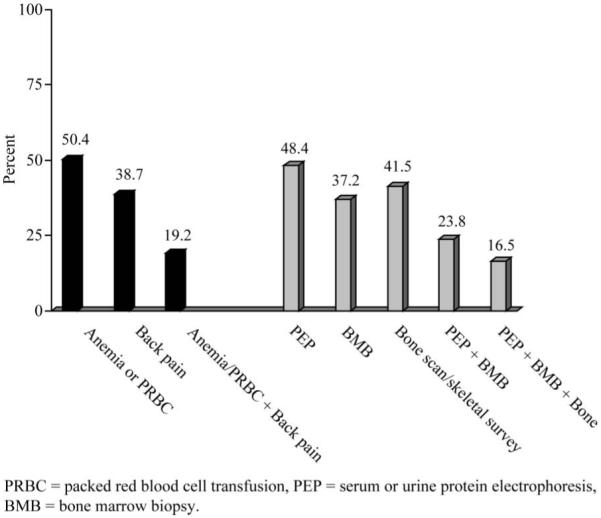
Frequency of pre-diagnosis signs, symptoms and procedures (n = 5483). The majority of study patients experienced anemia or had a transfusion of PRBCs prior to myeloma diagnosis. Less than 25% of patients had PEP and bone marrow biopsy prior to diagnosis.
Frequency and predictors of diagnostic delay
For the 3831 patients in the delay sample, 137 days (mean, SD 120, range 1–365) elapsed between the first claim for a myeloma sign or symptom and myeloma diagnosis; the median was 99 days (interquartile range=27–252). In more than 66% of the delay sample, the difference between the date of claim for a sign or symptom and myeloma diagnosis exceeded 30 days; subsequent analyses defined delay as exceeding the median number of days between sign or symptom and myeloma diagnosis. Table II shows the results from the ordered logistic regression model for estimating the likelihood of delay between sign or symptom and diagnosis, based on the quartile distribution. The presence of at least one comorbidity, in addition to both anemia/PRBC and back pain prior to myeloma diagnosis, was the strongest predictor of diagnostic delay (OR 1.6, 95% CI 1.3–2.0). However, the likelihood of delay was also high when comorbidity was present with only anemia or back pain (OR 1.4, 95% CI 1.2–1.6). Increased physician (OR 1.1, 95% CI 1.0–1.1) or hospital (OR 1.1, 95% CI 1.0–1.1) visits in the year preceding diagnosis increased the likelihood for delay. Other significant predictors of delay included the year of diagnosis, increasing age at the time of diagnosis, and non-white race/ethnicity. Males (OR 0.8, 95% CI 0.7–0.8) and patients initially diagnosed during an inpatient stay (OR 0.7, 95% CI 0.6–0.8) were significantly less likely to experience delay.
Table II.
Ordered logistic regression model predicting that the interval between first sign or symptom and diagnosis increased by quartile distribution (n = 3831)
| Odds ratio |
95% confidence interval |
|
|---|---|---|
| Male | 0.75 | 0.67–0.84 |
| Year of diagnosis | 1.03 | 1.01–1.06 |
| Age at diagnosis | 1.02 | 1.01–1.03 |
| Geographic region | ||
| Northeast | 0.92 | 0.78–1.09 |
| South | 0.98 | 0.81–1.20 |
| Midwest | 1.14 | 0.99–1.32 |
| Non-white race/ethnicity | 1.19 | 1.02–1.39 |
| No. of physician visits in past year | 1.05 | 1.05–1.06 |
| No. of inpatient stays in past year | 1.07 | 1.02–1.13 |
| Comorbidity: ≥1 present | ||
| Anemia/PRBC + back pain | 1.58 | 1.25–2.00 |
| No anemia/PRBC + back pain | 1.37 | 1.18–1.59 |
| Diagnosed during inpatient stay | 0.73 | 0.64–0.84 |
PRBC, packed red blood cell transfusion. Age at diagnosis measured for increasing year of age; Geographic regions were compared to West; non-white race/ethnicity was compared with white.
Predictors of post-diagnosis complications
After excluding 77 patients who had claims for renal or skeletal complications prior to myeloma diagnosis, 5406 patients were used to examine frequency and predictors of complications following myeloma diagnosis. Over a third of patients experienced at least one skeletal or renal complication (n = 1851); of these, 1160 patients had cord compression or fracture, and 796 experienced renal failure after diagnosis (105 patients experienced both). The results of the logistic regression model are shown in Table III. Patients initially diagnosed during an inpatient stay were more than more than twice as likely to experience a complication as those who were diagnosed as outpatients (95% CI 2.2–2.9). Additional significant predictors of complications included systemic chemotherapy within 6 months after diagnosis, and more inpatient stays in the year preceding diagnosis. Patients who had undergone bone marrow biopsy and PEP prior to diagnosis were significantly less likely to experience a complication (OR 0.6, 95% CI 0.5–0.6) than those who did not receive these diagnostic studies. We did not detect a significant effect of delay between sign or symptom and diagnosis on the likelihood of complications (OR 0.9, 95% CI 0.8–1.1). Our results did not change when considering a three-category variable of delay (no claim, delay and no delay), when we changed the measure of delay to meet or exceed the tenth percentile, or when an interaction term between comorbidity and delay was included (results not shown).
Table III.
Logistic regression model predicting renal or skeletal complication following diagnosis of multiple myeloma (n = 5406)
| Odds ratio |
(95% confidence interval) |
|
|---|---|---|
| Male | 1.00 | 0.89–1.12 |
| Year of diagnosis | 1.01 | 0.98–1.03 |
| Age at diagnosis | 1.00 | 0.99–1.01 |
| Geographic region | ||
| Northeast | 1.08 | 0.92–1.28 |
| South | 1.20 | 0.98–1.46 |
| Midwest | 1.02 | 0.88–1.19 |
| Non-white race/ethnicity | 0.94 | 0.80–1.10 |
| No. of physician visits in past year | 1.01 | 1.00–1.01 |
| No. of inpatient stays in past year | 1.06 | 1.01–1.12 |
| Comorbidity: ≥1 present | 1.09 | 0.95–1.24 |
| Diagnosed during inpatient stay | 2.53 | 2.22–2.88 |
| Bisphosphonate within 6 months after diagnosis |
1.08 | 0.93–1.25 |
| Chemotherapy within 6 months after diagnosis |
1.40 | 1.24–1.59 |
| BMB + PEP prior to diagnosis | 0.55 | 0.47–0.64 |
| Delay between sign or symptom and diagnosis |
0.91 | 0.80–1.03 |
BMB, bone marrow biopsy; PEP, serum or urine protein electrophoresis. Age at diagnosis measured for increasing year of age; Geographic regions were compared to West; non-white race/ethnicity was compared with white. Delay defined as exceeding sample median of time between myeloma diagnosis and sign or symptom.
Discussion
We found that Medicare enrollees in 13 regions of the United States experienced substantial variations in the timeliness of diagnostic work up for multiple myeloma. For example, for patients who visit a physician or hospital with signs or symptoms of myeloma, the time between the initial visit and definitive diagnosis ranges from 1 to 365 days, with mean of 137. Half of our study patients were diagnosed 99 or more days after the first visit. Male patients and patients diagnosed during an inpatient stay were significantly less likely to experience a delay of more than 99 days; however, non-white patients, patients with more physician visits and hospitalisations, and patients with comorbidities, were more likely to experience a delay as defined by our methods.
Nearly 20% of patients with any comorbidity, in addition to anemia and back pain, had a significantly greater likelihood of experiencing a delay in diagnosis of myeloma. Curiously, despite the presence of a sign or symptom often associated with myeloma, the diagnostic process appears to be more difficult when patients experience multiple medical problems, whether measured by actual number of comorbidities or heavy service utilisation. This may be because primary care providers are focussed on acute problems, and overlook signs and symptoms of myeloma. The lower likelihood of diagnostic delay for hospitalised patients may occur because multiple specialist consultants and more advanced diagnostic equipment are readily available in the inpatient setting, thus facilitating the completion and interpretation of diagnostic tests.
In our cohort, race was significantly associated with the timeliness of diagnostic workup that patients with myeloma received, affirming previous findings from other cancer patient populations [25]. The reasons for this are not clear and warrant further investigation; perhaps patients differ in their reporting of symptoms or may seek care in different ways based on race. In our analyses, females were more likely to experience diagnostic delay. However, because diagnostic delay is most frequently studied in gender-specific tumors, very little data are available on gender differences. One recent report that included colorectal and lung cancers found longer delays for women, but these differences were not statistically significant [25]. However our finding of increased diagnostic delays for females has also been reported in tuberculosis [39] and cardiovascular disease [40-42]. And despite more frequent diagnostic delays for women, research findings have shown increased distress from delays for women compared with men [43]. When comparing the effects of race and of gender on diagnostic delay for myeloma, the gender divide appears greater than the racial divide. Our data suggest it is difficult to sort the variance in diagnostic delay into discrete categories, such as patients, providers and health systems. Further study of interactions among these domains is warranted. Interestingly, despite the significant effect of race and gender on diagnostic delay, these factors were not implicated in the likelihood of complications.
We found no evidence for a direct relationship between delayed diagnosis and skeletal or renal complications. Rather, the primary predictors of complications were diagnoses made in the inpatient setting and administration of chemotherapy within 6 months of diagnosis; these factors may reflect poorer overall health status and higher severity of myeloma. When bone marrow biopsy and PEP were both performed close to the diagnosis date, the likelihood of complications was significantly reduced; however, less than 25% of patients had both procedures performed prior to diagnosis, and even fewer received additional radiographic evaluation for fracture. Thus, while it may often be assumed that diagnostic delays may pose harm to patients with cancer in general, our empirical findings do not show this to be the case for older adults with myeloma. Alternately, delays between diagnosis and treatment, or abnormal test results and treatment, may have significant associations with outcomes, and warrant further study.
Our finding of infrequent use of readily available diagnostic tests for myeloma is provocative given previous research findings that providers consider myeloma infrequently in the differential diagnosis of common signs and symptoms such as anemia and back pain [30]. A recent survey of primary care providers suggests that most are not familiar with PEP as a diagnostic test for myeloma [44]. Adoption of diagnostic guidelines [45] may enable providers to identify aggressive myeloma and initiate therapy earlier, which may have heightened significance as newer myeloma therapies have resulted in promising response rates [46,47].
Our data have limitations. First, they reflect a limited availability of data on the patient experience and actual clinical encounters. Second, we were not able to analyse total nor monoclonal protein levels, and clinicians may defer formal work up in patients with low levels, which would reflect smoldering myeloma [47]. Third, our data do not include a measure of corticosteroid administration, which is often used to initially stabilise myeloma and may affect skeletal complications. Fourth, our study is restricted to traditional Medicare enrollees who did not participate in managed care plans, which, despite including a large number of patients with myeloma, limits the generalisability of our findings. Replication of our analyses in populations where provider availability may be improved, such as health maintenance organisations or in the Veterans Health Administration, would help determine if provider factors are a contributing factor in diagnostic delay. Fifth, while the majority of patients with myeloma are diagnosed in older age, the benefits of newer agents have been more clearly demonstrated in younger patients who are eligible for transplantation [48]. Finally, no consensus exists for determining a clinically significant period of time between myeloma diagnosis and evaluation for a related sign or symptom. Thus, it is not yet clear how delay should be measured for analysis, and our use of the median time between sign or symptom for patients in the reported analyses may not be ideal; on the other hand, we estimated models using the mean, quartile categories, the tenth percentile, and a continuous measure, and obtained similar results.
In summary, while we did not see evidence of a relationship between diagnostic delay and complications for patients with multiple myeloma, we did observe significant differences in timeliness of care for patients by gender, race, and clinical complexity. Complications were, in general, most strongly associated with myeloma severity and overall health. Our data also suggest that reductions in complications might be achieved by performing bone marrow biopsy and PEP during the initial diagnostic workup, perhaps because the information obtained can be used to individualise treatments. Finally, the presence of multiple comorbidities may act to mask even signs and symptoms of myeloma, an unfortunate finding, as those patients with comorbidities are the ones most likely to need rapid diagnosis and treatment.
Acknowledgements
This work was supported by a training grant from the National Cancer Institute (R25 CA 057711-12) (Glorian Sorensen), and the Centers for Disease Control and Prevention (Grant #200-2002-00575, Task Order 21) (G.A.A.). Findings from this article were presented at the 2007 American Society for Hematology Annual Meeting, Atlanta, GA. This study used the linked SEER-Medicare database.
The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Programme, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services (IMS), Inc.; and the SEER programme tumor registries in the creation of the SEER-Medicare database. This project was supported by a grant from the Division of Cancer Prevention and Control of the Centers for Disease Control and Prevention (CDC) in Atlanta, GA; the findings and conclusions in this report do not necessarily represent that agency’s views. We thank Paul G. Richardson, MD, for his comments on this manuscript.
Appendix
Appendix 1.
Diagnosis and procedure codes used for analysis
| Variable type | Variable | ICD-9 diagnostic codes |
ICD-9 procedure codes |
CPT | HCPCS | BETOS | CEN | DRG |
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Multiple myeloma | 203.xx | ||||||
| Plasmacytoma | 238.6 | |||||||
| Exclusion | Amyloidosis | 277.3x | ||||||
| Sign or symptom |
Anemia codes | 280.0–280.1, 281.1–281.2, 283.xx, 285.2x, 285.9 |
||||||
| Back pain | 307.89, 724.2, 724.5 | |||||||
| PRBC transfusion | 99.03, 99.04 |
36430 | ||||||
| U/S PEP | 84156; 84165; 84181; 84182 |
|||||||
| Bone marrow biopsy | 41.31 | 20220, 20225, 20240, 20245, 85095, 85102 |
||||||
| Bone scan/skeletal survey |
92.14 | 76061, 76062, 78300, 78305, 78306, 78315, 78320 |
||||||
| Treatment | Bisphosphonates | J2430, J3487 | ||||||
| Complication | Skeletal Complication | 800–829 | ||||||
| Cord compression | 336.9 | |||||||
| Renal dysfunction | 580.xx-584.xx; 586 | |||||||
| Exclusion (pre-dx); Treatment (post-dx) |
XRT procedure Chemotherapy procedure |
V58.0, V66.1, V67.1 E930.7, E933.1, V58.1, V66.2, V67.2 |
92.2x 99.25 |
77xxx, 79xxx 964xx, 9651x, 9652x, 9653x, 9654x |
S8049 C1166, C1167, C1178, C9110, C9205, C9207, C9213-C9216, C9411, C9414-C9419, C942x, C9430-C9438, G0355, G0356, G0359-G0362, J7150, J85xx-J87xx, J8999, J9xxx, Q0083-Q0085, S9325-S9329, S933x-S937x, S9494-S9497 |
P7A O1D |
0330, 0333, 0339 0331, 0332, 0335 |
409 410 |
ICD-9, international classification of diseases, ninth revision; HCPCS, health care procedure coding system; CPT, current procedural terminology; BETOS, Berenson-Eggers type of service; CEN, revenue center code; DRG, diagnosis-related group; U/S PEP, urine or serum protein electrophoresis; PRBC, packed red blood cell.
References
- 1.Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press; Washington, DC: 2001. Committee on Quality of Health Care in America; Institute of Medicine. [Google Scholar]
- 2.United Kingdom Department of Health The NHS Improvement Plan: Putting People at the Heart of Public Services. 2004 June 24; [Internet] [cited 2008 March 1]. Available from: http://www.dh.gov/uk/publications.
- 3.Kohn LT, Corrigan JM, Donaldson MS, editors. To Err is Human: Building a Safer Health System. National Academy Press; Washington, DC: 2000. http://books.nap.edu/catalog/9728.html. [PubMed] [Google Scholar]
- 4.Jiwa M, Reid J, Handley C, Grimwood J, Ward S, Turner K, et al. Less haste more speed: factors that prolong the interval from presentation to diagnosis in some cancers. Fam Pract. 2004;21:299–303. doi: 10.1093/fampra/cmh314. [DOI] [PubMed] [Google Scholar]
- 5.Tabar L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, Grontoft O, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- 6.Arbman G, Nilsson E, Storgren-Fordell V, Sjodahl R. A short diagnostic delay is more important for rectal cancer than for colonic cancer. Eur J Surg. 1996;162:899–904. [PubMed] [Google Scholar]
- 7.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivotto IA, Gomi A, Bancej C, Brisson J, Tonita J, Kan L, et al. Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer. 2002;94:2143–2150. doi: 10.1002/cncr.10453. [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, Cinini C, Di Pietro C, Lombardi CP, Crucitti A, Bellantone R, et al. Diagnostic delay in breast cancer: correlation with disease stage and prognosis. Tumori. 1990;76:559–562. doi: 10.1177/030089169007600609. [DOI] [PubMed] [Google Scholar]
- 10.Robinson E, Mohilever J, Zidan J, Sapir D. Colorectal cancer: incidence, delay in diagnosis and stage of disease. Eur J Cancer Clin Oncol. 1986;22:157–161. doi: 10.1016/0277-5379(86)90025-8. [DOI] [PubMed] [Google Scholar]
- 11.DerKinderen DJ, Koten JW, Van Romunde LK, Nagelkerke NJ, Tan KE, Beemer FA, et al. Early diagnosis of bilateral retinoblastoma reduces death and blindness. Int J Cancer. 1989;44:35–39. doi: 10.1002/ijc.2910440107. [DOI] [PubMed] [Google Scholar]
- 12.United Kingdom Department of Health Faster and fairer access to cancer care – new cancer referral guidelines published. 2001 March 31; [press release]. [Internet] [cited 29 February 2008] Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Pressreleases/DH_4002515.
- 13.Hodgson TA, Buchanan JA, Garg A, Ilyas SE, Porter SR. An audit of the UK national cancer referral guidelines for suspected oral mucosal malignancy. Br Dent J. 2006;201:643–647. doi: 10.1038/sj.bdj.4814262. [DOI] [PubMed] [Google Scholar]
- 14.Mahon CC, Vaizey CJ, Taylor I, Boulos PB. Preliminary evaluation of United Kingdom National Referral Guidelines for lower gastrointestinal tract cancer. Colorectal Dis. 2002;4:111–114. doi: 10.1046/j.1463-1318.2002.00307.x. [DOI] [PubMed] [Google Scholar]
- 15.Panter SJ, Bramble MG, O’Flanagan H, Hungin AP. Urgent cancer referral guidelines: a retrospective cohort study of referrals for upper gastrointestinal adenocarcinoma. Br J Gen Pract. 2004;54:611–613. [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster JL, Sherman IW. Referral guidelines for cancer need closer scrutiny. BMJ. 2001;322:1491. [PMC free article] [PubMed] [Google Scholar]
- 17.Wender RC. Cancer screening and prevention in primary care. Obstacles for physicians. Cancer. 1993;72:1093–1099. doi: 10.1002/1097-0142(19930801)72:3+<1093::aid-cncr2820721326>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Williams PA, Williams M. Barriers and incentives for primary care physicians in cancer prevention and detection. Cancer. 1988;61:2382–2390. doi: 10.1002/1097-0142(19880601)61:11+<2382::aid-cncr2820611307>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Triezenberg DJ, Smith MA, Holmes TM. Cancer screening and detection in family practice: a MIRNET study. J Fam Pract. 1995;40:27–33. [PubMed] [Google Scholar]
- 20.Piga A, Graziano F, Zahra G, Cellerino R. Attitudes of non-oncology physicians dealing with cancer patients. A survey based on clinical scenarios in Ancona province, central Italy. Tumori. 1996;82:423–429. doi: 10.1177/030089169608200502. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson A, From L, Cohen A, Tipping J. Family physicians’ knowledge of malignant melanoma. J Am Acad Dermatol. 1997;37:953–957. doi: 10.1016/s0190-9622(97)70071-9. [DOI] [PubMed] [Google Scholar]
- 22.Canto MT, Horowitz AM, Drury TF, Goodman HS. Maryland family physicians’ knowledge, opinions and practices about oral cancer. Oral Oncol. 2002;38:416–424. doi: 10.1016/s1368-8375(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 23.Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92:1959–1970. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allgar VL, Neal RD. General practitioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients – Cancer. Eur J Cancer Care (Engl) 2005;14:409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 25.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the ‘National Survey of NHS Patients: Cancer’. Br J Cancer. 2005;92:1971–1975. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel GA, Friese CR, Magazu LS, Richardson LC, Fernandez ME, De Zengotita JJ, et al. Delays in referral and diagnosis for chronic hematologic malignancies: a literature review. Leuk Lymphoma. 2008;49:1352–1359. doi: 10.1080/10428190802124281. [DOI] [PubMed] [Google Scholar]
- 27.Barlogie B, Shaughnessy J, Epstein J, Sanderson R, Anaissie E, Walker R, et al. Plasma cell myeloma. In: Lichtman M, Beutler E, Kipps T, Seligsohn U, Kaushansky K, Prchal J, editors. Williams Hematology. 7th ed. McGraw-Hill; Columbus, OH: 2006. pp. 1501–1533. [Google Scholar]
- 28.Kariyawasan CC, Hughes DA, Jayatillake MM, Mehta AB. Multiple myeloma: causes and consequences of delay in diagnosis. QJM. 2007;100:635–640. doi: 10.1093/qjmed/hcm077. [DOI] [PubMed] [Google Scholar]
- 29.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Ong F, Hermans J, Noordijk EM, Wijermans PW, Kluin-Nelemans JC. Presenting signs and symptoms in multiple myeloma: high percentages of stage III among patients without apparent myeloma-associated symptoms. Ann Hematol. 1995;70:149–152. doi: 10.1007/BF01682035. [DOI] [PubMed] [Google Scholar]
- 31.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 32.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK. SEER cancer statistics review, 1973–1994, National Cancer Institute. 1997. NIH Publication 97–2789. [Google Scholar]
- 34.National Cancer Institute Number of persons by race and Hispanic ethnicity for SEER participants. 2000 [Internet] unknown [cited 2006 December 6] Available from: http://seer.cancer.gov/registries/data.html.
- 35.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 36.Kind S, Virnig B, McBean AM. SEER-Medicare: defining the date of diagnosis and treatment. 2007 December 3; [Internet] [cited 1 March 2008] Available from: http://healthservices.cancer.gov/seermedicare/considerations/date.html.
- 37.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosmer DW, Lemeshow S. Applied logistic regression. John Wiley & Sons, Inc.; New York, NY: 1989. p. 307. [Google Scholar]
- 39.Karim F, Islam MA, Chowdhury AMR, Johansson E, Diwan VK. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy Plan. 2007;22:329–334. doi: 10.1093/heapol/czm026. [DOI] [PubMed] [Google Scholar]
- 40.Meischke H, Larsen MP, Eisenberg MS. Gender differences in reported symptoms for acute myocardial infarction: impact on prehospital delay time interval. Am J Emerg Med. 1998;16:363–366. doi: 10.1016/s0735-6757(98)90128-0. [DOI] [PubMed] [Google Scholar]
- 41.Banks AD, Dracup K. Are there gender differences in the reasons why African Americans delay in seeking medical help for symptoms of an acute myocardial infarction? Ethn Dis. 2007;17:221–227. [PubMed] [Google Scholar]
- 42.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, et al. National Registry of Myocardial Infarction I. Sex and racial differences in the management of acute myocardial infarction, 1994–2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risberg T, Sorbye SW, Norum J, Wist EA. Diagnostic delay causes more psychological distress in female than in male cancer patients. Anticancer Res. 1996;16:995–999. [PubMed] [Google Scholar]
- 44.Rosen PJ, Wender RC, Kadkhoda H, Kober SL. Measuring the ability of primary-care physicians to diagnose and manage patients with hematologic malignancies; ASH Annual Meeting Abstracts; 2007.p. 3312. [Google Scholar]
- 45.National Comprehensive Cancer Network Clinical practice guideline for multiple myeloma. 2008 August 14; [Internet] [cited 1 March 2008] Available from: http://www.nccn.org.
- 46.Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 47.Anargyrou K, Dimopoulos MA, Sezer O, Terpos E. Novel anti-myeloma agents and angiogenesis. Leuk Lymphoma. 2008;49:677–689. doi: 10.1080/10428190701861686. [DOI] [PubMed] [Google Scholar]
- 48.San-Miguel J, Harousseau JL, Joshua D, Anderson KC. Individualizing treatment of patients with myeloma in the era of novel agents. J Clin Oncol. 2008;26:2761–2766. doi: 10.1200/JCO.2007.15.2546. [DOI] [PubMed] [Google Scholar]


