Abstract
Although arachidonoyl ethanolamide (AEA or anandamide) is the first identified endocannabinoid, its roles in synaptic signaling and neuronal survival are still controversial. Here we report that AEA induced a dose-dependent elevation of the frequency of miniature excitatory postsynaptic currents (mEPSCs) in mouse hippocampal neurons in culture. This potentiation was not blocked by SR141716 or AM251, selective cannabinoid receptor antagonists), indicating that the AEA elevation of mEPSCs is not mediated via the CB1 receptor. Similarly, capsazepine and iodoresiniferatoxin, selective vanilloid receptor antagonists, and ryanodine also failed to inhibit the effect of AEA on mEPSCs. However, 2-APB and Xestospongin C, IP3 inhibitors, significantly attenuated AEA-induced increase in hippocampal excitatory synaptic transmission. Application of 3-deoxy-3-fluoro-D-myo-inositol 1,4,5-trisphosphate enhanced the frequency of mEPSCs and occluded the effect of AEA on mEPSCs. Our results suggest that AEA-produced stimulatory effect on excitatory glutamatergic synaptic transmission is likely mediated via an IP3 pathway.
Keywords: endocannabinoids, cannabinoid receptors, vanilloid receptor, ryanodine receptor, excitatory postsynaptic currents, hippocampus
Endocannabinoids (eCBs) are endogenous signaling mediators and have been demonstrated to be involved in a variety of physiological, pharmacological and pathological processes (Alger, 2002; Bisogno et al., 2008; Chevaleyre et al., 2006; Cinar et al., 2008; Freund et al., 2003; Hájos and Freund, 2002; Mackie, 2006; Páldyová et al., 2008; Páldy et al., 2008; Piomelli, 2003; Sarne and Mechoulam, 2005; van der Stelt and Di Marzo, 2005). Arachidonoyl ethanolamide (AEA or anandamide) is the first identified endogenous ligand for G protein-coupled cannabinoid receptors (Devane et al., 1992). Despite a similar chemical structure of AEA and second endogenous ligand for the cannabinoid receptor, 2-arachidonoyl glycerol (2-AG) (Mechoulam et al., 1995; Stella et al., 1997), they have very different pathways for their synthesis and degradation (Freund et al., 2003; Kozak et al., 2004; Mackie, 2006; Piomelli, 2003; Sang and Chen, 2006; Sugiura et al., 2006). For instance, 2-AG is mainly produced from diacylglycerol (DAG) by diacylglycerol lipase (DGL) and hydrolyzed to arachidonic acid (AA) by monoacylglycerol lipase (MGL), whereas AEA is largely synthesized from N-arachidonoylphosphatidylethanolamine (NAPE) by phospholipase D (PLD) and degraded to AA by fatty acid amide hydrolase (FAAH). Also the enzymes that synthesize 2-AG are present in postsynaptic dendritic spines (Katona et al., 2006; Yoshida et al., 2006), while the enzymes that make AEA appear to be present in presynaptic terminals (Nyilas et al., 2008). In addition, AEA is a partial CB1 receptor and a weak CB2 receptor agonist, and an agonist for the vanilloid receptor (De Petrocellis and Di Marzo, 2005; Ross, 2003; van der Stelt & Di Marzo; 2004; Zygmunt et al., 1999), whereas 2-AG is a full agonist for both CB1 and CB2 receptors (Bisogno et al., 2005; Freund et al., 2003; Piomelli, 2003; Sugiura et al., 2006). In particular, 2-AG protects neurons from harmful insults (Panikashvili et al., 2001; 2005; 2006; Gopez, et al., 2005), whereas AEA exhibits paradox actions, i.e., inducing neurotoxicity and neuroprotection (Marsicano et al., 2003; Movsesyan et al., 2004; Cernak et al., 2004; Sarne & Mechoulam, 2005). This means that there are undefined mechanisms in AEA-mediated signaling events in synaptic activity and neuronal survival. Here, we demonstrate that AEA produced a dose-dependent potentiation of excitatory glutamatergic synaptic transmission, which has not been reported before. This potentiation was not blocked by CB1, vanilloid and ryanodine receptor antagonists, but significantly attenuated by IP3 antagonists and mimicked by an IP3 agonist. Our results suggest that AEA elevates presynaptic probability release of excitatory neurotransmitter glutamate via an IP3 pathway.
Methods
Primary hippocampal neuron culture
Primary hippocampal neurons from mouse pups (P0 to P1) were cultured as described previously (Sang et al., 2005; 2006; 2007; Zhang and Chen, 2008), according to the guidelines approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center. Briefly, hippocampi were dissected out from pups under microscope and triturated in serum-free culture medium after meninges were removed. Tissue was incubated in oxygenated trypsin for 10 minutes at 37°C and then mechanically triturated. Cells were spun down and resuspended in Neurobasal/B27 medium (Invitrogen) supplemented with 0.5 mM L-glutamine, penicillin/ streptomycin and 25 μM glutamate. Cells (1 × 106) were loaded into poly-D-lysine-coated 35-mm culture dishes for electrophysiological recordings. Medium was changed every three days with the same medium without glutamate until use. The extent of astroglial cells in the culture was ~2 to 5% at 10 days in vitro (DIV) estimated by staining with NeuN, a neuronal marker, GFAP, an astrocytic marker, and OX-42, a microglial marker in conjunction with the DAPI staining as previously described (Sang et al., 2005). Cultures were used between 10-21 DIV.
Electrophysiological recordings
Miniature Excitatory postsynaptic currents (mEPSCs) were recorded in hippocampal neurons in culture under voltage clamp using an Axopatch-200B amplifier as described previously (Sang et al, 2005; 2006; 2007). Recording pipettes (4-5 MΩ) were pulled from borosilicate glass with a micropipette puller (Sutter Instrument). The internal pipette solution contained (in mM) 115.0 Cs gluconate, 15.0 CsCl, 4.0 NaCl, 10.0 HEPES, 0.5 EGTA, 4.0 Mg2ATP, and 0.5 Na2GTP (pH 7.25 with CsOH). The membrane potential was held at −70 mV. The external solution contained (in mM): 130.0 NaCl, 2.5 KCl, 1.0 MgCl2, 10.0 HEPES, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose (pH 7.4 with NaOH). To isolate mEPSCs, tetrodotoxin (TTX, 0.5 to 1 μM), a voltage-gated Na+ channel blocker, and bicuculline (10 μM), a GABAA receptor blocker, were included in the external solution. All experiments were performed at room temperature (22~24°C). The frequency, amplitude and kinetics of mEPSCs were analyzed using the MiniAnalysis program.
Chemicals and drugs
2-AG and AEA were purchased from Cayman Chemical (Ann Arbor, MI). These chemicals were dissolved in ethanol to make stock solutions at concentrations of 20 mM and distributed in small vials, and were diluted with the external solution to desired concentrations just before experiments. They were applied by lowering the pipette (~50 μm of tip size) to within 30 to 50 μm of the recorded cell, and the application was terminated by removal of the pipette from the bathing medium. 2-APB, AM251, capsazepine and ryanodine (Tocris, Ellisville, MO), SR141716 (Provided by Chemical Synthesis and Drug Supply Program, the National Institute of Mental Health) were dissolved in DMSO to make up stock solutions at concentrations of 50 to 100 mM, and applied through bath perfusion. The final concentration of DMSO was 0.01%. All other drugs and chemicals were obtained from Sigma (St. Louis, MO), unless stated otherwise. To rule out potentially nonspecific effects of the solvents, same amount of ethanol or DMSO was included in the control external solution.
Data analysis
Data are presented as mean ± S.E.M. Unless stated otherwise, Student's t-test was used for comparisons of before and after drug applications, analysis of variance (ANOVA) followed by Student-Newman-Keuls post-hoc test were used for between group comparisons, and the Kolmogorov-Smirnov test was used for comparisons of mEPSC distribution. Differences were considered significant when P< 0.05.
Results
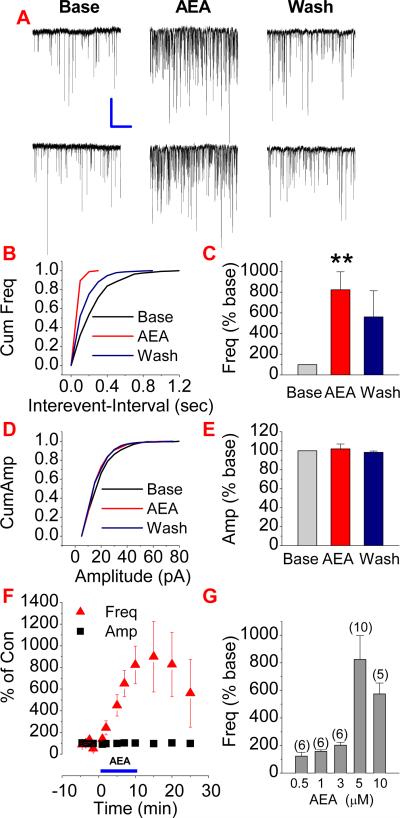
Recently, we demonstrated that 2-AG produces a significant reduction of the frequency of mEPSCs in cultured hippocampal neurons (Sang et al., 2007). To determine whether AEA is also capable of suppressing miniature activity in hippocampal neurons, we examined the effect of AEA on mEPSCs in mouse hippocampal neurons in culture. AEA was applied by lowering the pipette (~50 μm of tip size) to within 30 to 50 μm of the recorded cell, and the application was terminated by removal of the pipette from the bathing medium. As shown in Figure 1, AEA produced a dose dependent increase in the frequency of mEPSCs, but not the amplitude, suggesting a presynaptically mediated effect. This is opposite to that of 2-AG as reported previously (Melis et al., 2004; Sang et al., 2007; Straiker and Mackie, 2005). Meanwhile, we analyzed kinetics of mEPSCs in terms of rising and decay time constants, and did not find significant changes in kinetics of mEPSCs.
Figure 1.
Anandamide potentiates excitatory synaptic transmission in hippocampal neurons in culture. (A). Representative sweeps of miniature excitatory postsynaptic currents (mEPSCs) in the absence or presence of AEA (5 μM) and washout. Miniature EPSCs were recorded in primary hippocampal neurons in culture from 10 to 15 DIV. The membrane potential was held at −70 mV. Bicuculline (10 μM) and TTX (0.5 to 1 μM) were included in the external solution. The synaptic events were analyzed using the MiniAnalysis program. (B). Cumulative probability of mEPSC frequency recorded in neurons in the absence and presence of AEA and washout. (C). Mean percentage changes in the frequency of mEPSCs (n=10). (D). Cumulative probability of mEPSC amplitude, and (E). Mean percentage changes in the amplitude of mEPSCs. (F). Time courses of AEA-induced changes in frequency and amplitude of mEPSCs. (G). AEA induces a dose dependent decrease in the frequency of mEPSCs. **<P<0.01 compared with baseline, Scale bar: 20pA/2sec.
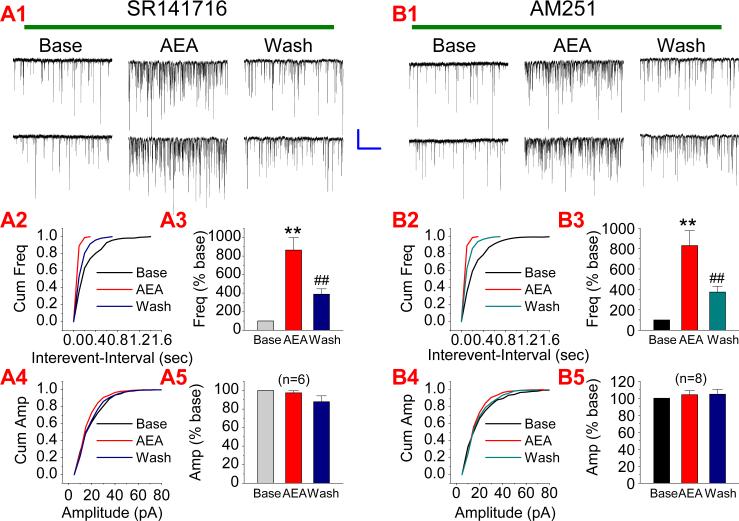
The action of eCBs in the brain is mainly mediated via the CB1 receptor. To examine whether the CB1 receptor mediates AEA elevation of mEPSCs, we used SR141716 (SR), a selective CB1 receptor antagonist. Neurons were treated with SR (1 μM) via bath application at least for 30 min. As shown in Figure 2A, AEA (5 μM) still enhanced mEPSCs in the presence of SR (n=6, p<0.01), suggesting that the AEA potentiation of excitatory synaptic transmission is not mediated via the CB1 receptor. To confirm this, we employed another selective CB1 receptor antagonist, AM251. It appears that AM251 failed to prevent AEA-elevated mEPSCs (n=8, p<0.01) (Figure 2B). These results provide important information that AEA-produced enhancement of excitatory synaptic transmission is not mediated via the CB1 receptor.
Figure 2.
AEA-induced increase in the frequency of mEPSCs is not mediated via a CB1 receptor. (A1). Representative sweeps of mEPSCs in neurons treated with SR141716 (1 μM) in the absence or presence of AEA (5 μM) and washout. (A2). Cumulative probability of mEPSC frequency. (A3). Mean percentage changes in the frequency of mEPSCs (n=6). (A4). Cumulative probability of mEPSC amplitude, and (A5). Mean percentage changes in the amplitude of mEPSCs. (B1). Representative sweeps of mEPSCs in neurons treated with AM 251 (1 μM) in the absence or presence of AEA (5 μM) and washout. (B2). Cumulative probability of mEPSC frequency. (B3). Mean percentage changes in the frequency of mEPSCs (n=8). (B4). Cumulative probability of mEPSC amplitude, and (B5). Mean percentage changes in the amplitude of mEPSCs. **P<0.01 compared with baseline; ##P<0.01 compared with AEA. Scale bar: 20pA/2sec
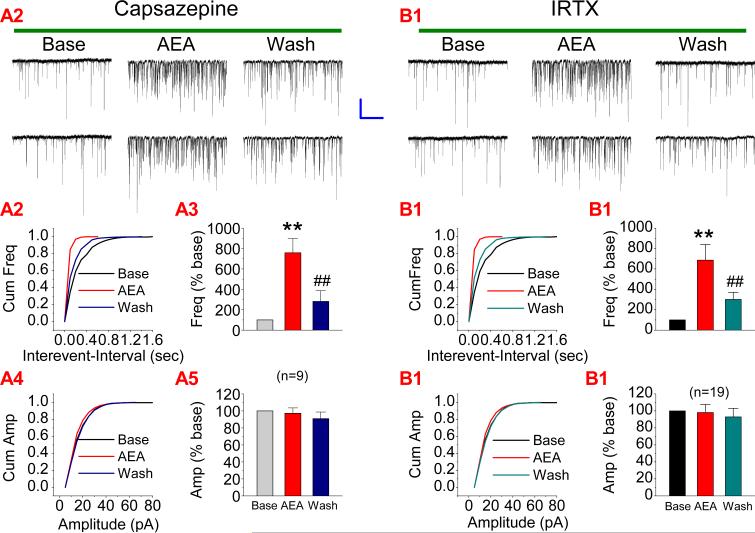
Different from 2-AG, AEA also is an agonist for the vanilloid receptors (De Petrocellis and Di Marzo, 2005; Ross, 2003; van der Stelt & Di Marzo; 2004; Zygmunt et al., 1999). Therefore, we decided to use a vanilloid receptor antagonist, capsazepine, to determine whether the effect of AEA on mEPSCs is mediated by the vanilloid receptors. As indicated in Figure 3A, bath application of capsazepine (10 μM) still failed to block AEA-induced increase mEPSCs (n=9, p<0.01), suggesting that the effect is not mediated via the vanilloid receptors. It has been demonstrated that AEA-induced increases mEPSCs in dopaminergic neurons of the rat substantia nigra is mediated via the TRPV1 receptors (Marinelli et al., 2003). To determine whether AEA-elevated mEPSCs in hippocampal neurons is mediated via the TRPV1 receptor, we used iodoresiniferatoxin (IRTX), a potent and selective antagonist of TRPV1 receptors. As shown in Figure 3B, IRTX (3 nM) did not prevent the AEA-induced increase in the frequency of mEPSCs in hippocampal neurons in culture (n=19, p<0.01), indicating that the TRPV1 receptor does not mediated the effect of AEA in hippocampal neurons.
Figure 3.
AEA-induced increase in excitatory synaptic transmission is not mediated via a vanilloid receptor. (A1). Representative sweeps of mEPSCs in neurons treated with capsazepine (vanilloid receptor antagonist, 10 μM) in the absence or presence of AEA (5 μM) and washout. (A2). Cumulative probability of mEPSC frequency. (A3). Mean percentage changes in the frequency of mEPSCs (n=9). (A4). Cumulative probability of mEPSC amplitude, and (A5). Mean percentage changes in the amplitude of mEPSCs. (B1). Representative sweeps of mEPSCs in neurons treated with iodoresiniferatoxin (IRTX, selective TRPV1 receptor antagonist, 3 nM) in the absence or presence of AEA (5 μM) and washout. (B2). Cumulative probability of mEPSC frequency. (B3). Mean percentage changes in the frequency of mEPSCs (n=19). (B4). Cumulative probability of mEPSC amplitude, and (B5). Mean percentage changes in the amplitude of mEPSCs. **P<0.01 compared with baseline; ##P<0.01 compared with AEA. Scale bar: 20pA/2sec
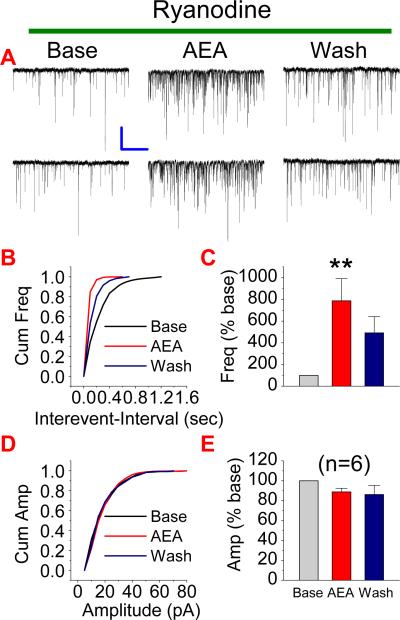
Endogenous cannabinoid-mediated depolarization-induced suppression of inhibition (DSI) has been suggested to be associated with mobilizing intracellular Ca2+ through ryanodine receptors (Isokawa and Alger, 2005). Although this process is likely to facilitate release of eCBs, we wondered whether AEA-induced potentiation of excitatory synaptic transmission is via the ryanodine receptor, which elevates intracellular Ca2+, resulting in enhanced synaptic release of glutamate. To this end, we treated neurons with ryanodine (20 μM) at least for 20 min to block the ryanodine receptors. As shown in Figure 4, AEA (5 μM) still potentiated mEPSCs in the presence of ryanodine (n=6, p<0.01). This means that ryanodine receptors are not involved in the AEA-produced increase of synaptic activity.
Figure 4.
Ryanodine receptor does not mediate the AEA-induced effect on mEPSCs. (A). Representative sweeps of mEPSCs in neurons treated with ryanodine (ryanodine receptor antagonist, 20 μM) in the absence or presence of AEA (5 μM) and washout. (B). Cumulative probability of mEPSC frequency. (C). Mean percentage changes in the frequency of mEPSCs (n=6). (D). Cumulative probability of mEPSC amplitude, and (E). Mean percentage changes in the amplitude of mEPSCs. **P<0.01 compared with baseline. Scale bar: 20pA/2sec
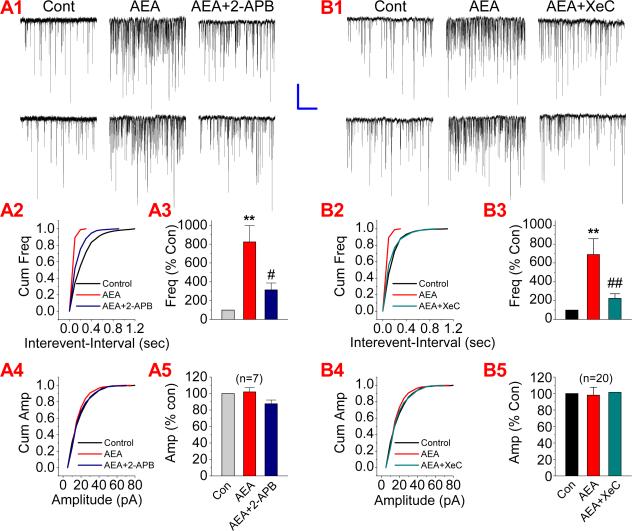
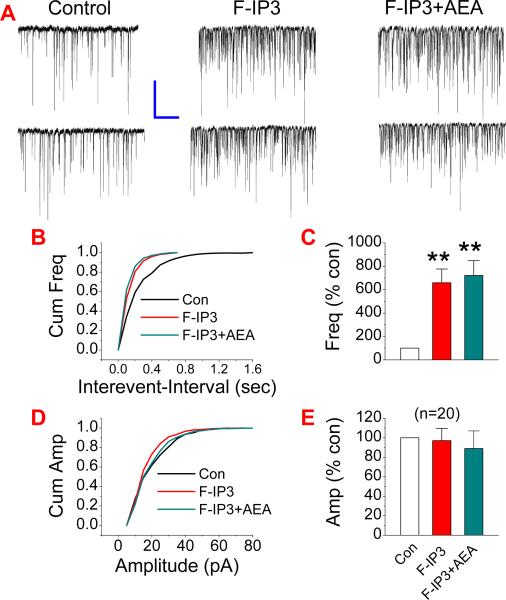
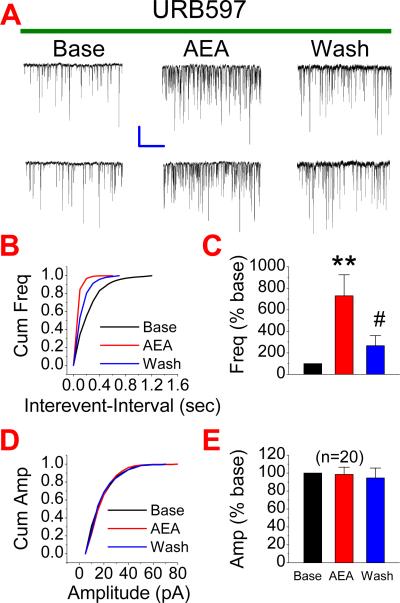
A rise of intracellular Ca2+ is critical for synaptic release of neurotransmitters. Since TTX that prevents neuronal firing was included in the bath solution, it is unlikely that presynaptic voltage-dependent Ca2+ channels were activated during recordings. We speculated that IP3 pathway, which mobilizes intracellularly stored Ca2+ release, might be involved in the AEA-elevated synaptic release of glutamate. To test this idea, we first used 2-APB, a selective IP3 receptor antagonist. As shown in Figure 5A, AEA-produced potentiation of mEPSCs (n=7, p<0.01) was significantly attenuated in neurons treated with 2-APB (20 μM) (n=7, p<0.05). To confirm the involvement of the IP3 pathway in the AEA-induced effect on mEPSCs, we adopted another selective IP3 inhibitor, Xestospongin C (XeC). Similar to that of 2-APB, application of XeC (1 μM) blocked the AEA-enhanced mEPSCs (n=20, p<0.01 for AEA; n=20, ##<0.01 for AEA in the presence of XeC) (Fig. 5B). To further ascertain the role of the IP3 signaling pathway in the AEA-induced effect on excitatory synaptic transmission, we applied 3-deoxy-3-fluoro-D-myo-inositol 1,4,5-trisphosphate (F-IP3), an IP3 agonist. As shown in Figure 6, administration of F-IP3 (50 μM) significantly elevated the frequency of mEPSCs (n=20, p<0.01), mimicking the effect of AEA. The presence of F-IP3 also occluded the effect of AEA on the frequency of mEPSCs (n=20, p<0.01). To exclude the possibility that the AEA-induced effect on mEPSCs results from its metabolite arachidonic acid, which has been shown to elevate spontaneous glutamate synaptic transmission in rat substantia gelatinosa neurons (Yue et al., 2005), we included URB597, an inhibitor of FAAH that hydrolyzes AEA to arachidonic acid, in the bath solution. As shown in Figure 7, AEA still increased the frequency of mEPSCs (n=20, p<0.01) in the presence of URB597 (1 μM). These results indicate that the IP3 signaling pathway likely mediates the AEA-produced increase in excitatory synaptic transmission.
Figure 5.
AEA-induced elevation of mEPSCs is mediated via an IP3 pathway. (A1). Representative sweeps of mEPSCs recorded in hippocampal neurons treated with and without 2-APB (IP3 receptor antagonist, 20 μM) in the absence or presence of AEA (5 μM). (A2). Cumulative probability of mEPSC frequency. (A3). Mean percentage changes in the frequency of mEPSCs (n=7). (A4). Cumulative probability of mEPSC amplitude, and (A5). Mean percentage changes in the amplitude of mEPSCs. (B1). Representative sweeps of mEPSCs recorded in hippocampal neurons treated with and without Xestospongin C (XeC, a potent and selective IP3 receptor antagonist, 1 μM) in the absence or presence of AEA (5 μM). (B2). Cumulative probability of mEPSC frequency. (B3). Mean percentage changes in the frequency of mEPSCs (n=20). (B4). Cumulative probability of mEPSC amplitude, and (B5). Mean percentage changes in the amplitude of mEPSCs. **<P<0.01 compared with baseline; #P<0.05, compared with AEA. Scale bar: 20pA/2sec
Figure 6.
Activation of the IP3 enhances the frequency of mEPSCs. (A). Representative sweeps of mEPSCs in the absence or presence of 3-deoxy-3-fluoro-D-myo-inositol 1,4,5-trisphosphate (F-IP3, 50 μM) and F-IP3+AEA (5 μM). (B). Cumulative probability of mEPSC frequency. (C). Mean percentage changes in the frequency of mEPSCs (n=20). (D). Cumulative probability of mEPSC amplitude, and (E). Mean percentage changes in the amplitude of mEPSCs. **P<0.01 compared with baseline. Scale bar: 20pA/2sec
Figure 7.
Inhibition of fatty acid amide hydrolase does not prevent the AEA-induced effect on mEPSCs. (A). Representative sweeps of mEPSCs in neurons treated with URB597 (selective inhibitor of FAAH, 1 μM) in the absence or presence of AEA (5 μM) and washout. (B). Cumulative probability of mEPSC frequency. (C). Mean percentage changes in the frequency of mEPSCs (n=20). (D). Cumulative probability of mEPSC amplitude, and (E). Mean percentage changes in the amplitude of mEPSCs. **P<0.01 compared with baseline; # P<0.05 compared with AEA. Scale bar: 20pA/2sec
DISCUSSION
In the present study, we provide important evidence that AEA potentiates excitatory synaptic transmission in a dose-dependent manner in cultured hippocampal neurons. The effect is not mediated by CB1, vanilloid, and ryanodine receptors, but by an IP3 receptor. This information suggests that AEA may not only have an inhibitory, but also a stimulatory effect on excitatory glutamatergic synaptic transmission.
Several lines of evidence show that eCBs are involved in a variety of physiological and pharmacological functions. In particular, eCBs play important roles in synaptic signaling and neuronal survival (Freund et al., 2003; Piomelli, 2003; Chevaleyre et al., 2006; Mackie, 2006; Eljaschewitsch et al., 2006; Gopez et al., 2005; Marsicano et al., 2003; Melis et al., 2006; Panikashvili et al., 2001; 2005). Nevertheless, functional roles of eCBs in synaptic activity and neuronal protection are still controversial. For instance, eCBs mainly exert an inhibitory effect on both GABAergic and glutamatergic synaptic transmission (Freund et al., 2003; Piomelli, 2003; Chevaleyre et al., 2006; Mackie, 2006). However, eCBs have also been demonstrated to facilitate excitatory synaptic transmission (Carlson et al., 2002; Chevaleyre and Castillo, 2004; Zhu & Lovinger, 2007). Similarly, available evidence shows that eCBs protect neurons from harmful insults (Eljaschewitsch et al., 2006; Gopez et al., 2005; Marsicano et al., 2003; Melis et al., 2006; Panikashvili et al., 2001; 2005; Sarne and Mechoulam, 2005; van der Stelt and Di Marzo, 2005; Zhang & Chen, 2008). However, eCBs also produce neurotoxic effects (Sarne and Mechoulam, 2005; van der Stelt and Di Marzo, 2005; Movsesyan et al., 2004; Cernak et al., 2004). In particular, AEA has been shown to exhibit neuroprotective and neurotoxic effects. This means that there are undefined mechanisms in mediating eCBs in synaptic modification and neuronal survival. The observations made from the present study that AEA elevates synaptic release of glutamate may be responsible for eCB-mediated enhancement of excitatory synaptic transmission and production of neurotoxicity.
AEA and 2-AG are the most studied eCBs. However, they exhibit different functional roles in synaptic transmission and neuronal survival. Early studies show that both AEA and 2-AG might act as retrograde messengers in modulating synaptic transmission, which is demonstrated by DSI or DSE (Freund et al., 2003). Increasing evidence suggests that 2-AG may be a retrograde signaling molecule in CB1 receptor-dependent DSI or DSE (Chevaleyre et al., 2006; Alger, 2005; Kim & Alger, 2004; Mackie, 2006; Melis et al., 2004; Makara et al., 2005; Safo and Regehr, 2005; Straiker & Mackie, 2005; Szabo et al., 2006; Heifets & Castillo, 2009). We found recently that 2-AG produces a dose-dependent inhibition of mEPSCs (Sang et al., 2007), while AEA observed in the present study produces a dose-dependent potentiation of mEPSCs. These results provide new information indicating that the role of AEA and 2-AG in synaptic signaling is different. However, it is still not clear whether the contribution of AEA to neurotoxicity is also mediated via an IP3 pathway, which warrants for further investigation.
Actions of exogenous and endogenous cannabinoids on synaptic transmission are primarily mediated through neuronal CB1 receptors. In the present study, we found that selective CB1 receptor antagonists fail to block the AEA potentiation of mEPSCs, suggesting an involvement of an orphan receptor in the AEA-mediated effect. Accumulated information suggests that there may be additional cannabinoid receptors with signaling distinct from CB1 and CB2 receptors (Hájos et al., 2001; Mackie and Stella, 2006; Ryberg et al., 2007; Lauckner et al., 2008). Recent evidence shows that GRP55 may be a cannabinoid receptor (Mackie and Stella, 2006; Ryberg et al., 2007). This receptor does not couple to Gi/o, instead, it couples to Gq and phospholipase C. Interestingly, activation of this receptor increases intracellular Ca2+ via an IP3 receptor (Lauckner et al., 2008). At present study, we are not able to define whether the AEA-mediated increase in mEPSC is mediated via the GRP55, but our data provide the information that the AEA potentiation of synaptic transmission is not mediated via the CB1, vanilloid, and ryanodine receptors, but via an IP3 pathway.
ACKNOWLEDGMENTS
The authors thank NIH NIMH Chemical Synthesis and Drug Supply Program for providing SR141716. This work was supported by National Natural Science Foundation of P R China (20607013 and 20877050), Natural Science Foundation of Shanxi Province (2009011049-3 and 20051043), Scientific Research Foundation for the Returned Overseas Chinese Scholars of Shanxi, and Program for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi (to NS); and Unite States Public Health Service Grant R01NS054886 from National Institute of Neurological Disorders and Stroke, National Institutes of Health (to CC).
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signaling system: biochemical aspects. Pharmacol. Biochem. Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Martire A, Petrosino S, Popoli P, Di Marzo V. Symptom-related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington's disease. Neurochem Int. 2008;52:307–313. doi: 10.1016/j.neuint.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Ann. Rev. Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cernak I, Vink R, Natale J, Stoica B, Lea PM, IV, Movsesyan V, Ahmed F, Knoblach SM, Fricke ST, Faden AI. The “dark side” of endocannabinoids: a neurotoxic role for anandamide. J. Cereb. Blood Flow Metab. 2004;24:564–578. doi: 10.1097/00004647-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Cinar R, Freund TF, Katona I, Mackie K, Szucs M. Reciprocal inhibition of G-protein signaling is induced by CB(1) cannabinoid and GABA(B) receptor interactions in rat hippocampal membranes. Neurochem Int. 2008;52:1402–1409. doi: 10.1016/j.neuint.2008.02.005. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci. 2005;77:1651–1666. doi: 10.1016/j.lfs.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Eljaschewitsch E, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gopez JJ, et al. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neurosci. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem. Phys. Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, Alger BE. Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell. J. Physiol. 2005;567:1001–1010. doi: 10.1113/jphysiol.2005.094219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat. Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JL, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr. Pharmaceut. Design. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen H-Y, Lu H-C, Hille B, Mackie K. GRP55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. Am. Assoc. Pharm. Sci. J. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J. Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, et al. Protective activation of the endocannabinoid system during ischemia in dopamine neurons. Neurobiol. Dis. 2006;24:15–27. doi: 10.1016/j.nbd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Movsesyan VA, Stoica BA, Yakovlev AG, Knoblach SM, Lea PM, IV, Cernak I, Vink R, Faden AI. Anandamide-induced cell death in primary neuronal cultures: role of calpain and caspase pathways. Cell Death Differ. 2004;11:1121–1132. doi: 10.1038/sj.cdd.4401442. [DOI] [PubMed] [Google Scholar]
- Nyilas R, Dudok B, Urbán GM, Mackie K, Watanabe M, Cravatt BF, Freund TF, Katona I. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci. 2008;28:1058–1063. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:427–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J. Cereb. Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, Shohami E. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Páldyová E, Bereczki E, Sántha M, Wenger T, Borsodi A, Benyhe S. Noladin ether, a putative endocannabinoid, inhibits mu-opioid receptor activation via CB2 cannabinoid receptors. Neurochem Int. 2008;52:321–328. doi: 10.1016/j.neuint.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Páldy E, Bereczki E, Sántha M, Wenger T, Borsodi A, Zimmer A, Benyhe S. CB(2) cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: Role of CB(2) receptor in pain. Neurochem Int. 2008;53:309–316. doi: 10.1016/j.neuint.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, et al. The orphan receptor GRP55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr. Drug Target- CNS Neurol. Dis. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J. Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. Prostaglandin E2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates hippocampal inhibitory synaptic transmission. J. Physiol. (Lond) 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Chen C. Lipid signaling and synaptic plasticity. Neuroscientist. 2006;12:425–434. doi: 10.1177/1073858406290794. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory Glutamatergic Synaptic Transmission and Induces Neurotoxicity. J. Neurochem. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J. Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog.Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J. Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stelt M, Di Marzo V. Endovanilloids: Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. NeuroMolecular. Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Fujita T, Kumamoto E. Phospholipase A2 activation by melittin enhances spontaneous glutamatergic excitatory transmission in rat substantia gelatinosa neurons. Neurosci. 2005;135:485–495. doi: 10.1016/j.neuroscience.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem. 2008;283:22601–22611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]