Abstract
An increase in ipsilateral descending motor pathway activity has been reported following hemiparetic stroke. In axial muscles, increased ipsilateral cortical activity has been correlated with good recovery whereas in distal arm muscles it is correlated with poor recovery. Currently, little is known about the control of proximal upper limb muscles following stroke. This muscle group is less impaired than the distal arm muscles following stroke, yet contributes to the abnormal motor coordination patterns associated with movements of the arm which can severely impair reaching ability. This study used transcranial magnetic stimulation (TMS) to evaluate the presence and magnitude of ipsilateral and contralateral projections to the pectoralis major (PMJ) muscle in stroke survivors. A laterality index (LI) was used to investigate the relationship between ipsilateral and contralateral projections and strength, clinical impairment level, and the degree of abnormal coordination expressed in the arm. The ipsilateral and contralateral hemispheres were stimulated using 90% TMS intensity while the subject generated shoulder adduction torques in both arms. Motor evoked potentials (MEPs) were measured in the paretic and non-paretic PMJ. The secondary torque at the elbow was measured during maximal adduction as an indicator of the degree of extensor synergy. Ipsilateral MEPs were most common in stroke survivors with moderate to severe motor deficits. The LI was correlated with clinical impairment level (P = 0.05) and the degree of extension synergy expressed in the arm (P = 0.03). The LI was not correlated with strength. These results suggest that increased excitability of ipsilateral pathways projecting to the proximal upper arm may contribute to the expression of the extension synergy following stroke. These findings are discussed in relation to a possible unmasking or upregulation of oligosynaptic cortico-bulbospinal pathways following stroke.
Keywords: Stroke, Synergy, Transcranial magnetic stimulation, Abnormal, Coordination, Strength
Introduction
After a stroke that has resulted in functional motor impairment of the contralateral upper limb, there is frequently an increase in the activity of motor cortical regions in the hemisphere ipsilateral to the paretic arm. This finding has been demonstrated using functional magnetic resonance imaging (fMRI) (Cao et al. 1998; Carey et al. 2002; Cramer et al. 1997; Feydy et al. 2002; Marshall et al. 2000; Ward et al. 2003), positron emission tomography (PET) (Chollet et al. 1991; Honda et al. 1997; Weiller et al. 1993), electroencephalography (EEG) (Green et al. 1999; Kopp et al. 1999; Serrien et al. 2004; Verleger et al. 2003) and transcranial magnetic stimulation (TMS) (Alagona et al. 2001; Chen et al. 2003; Fujiwara et al. 2001; Strens et al. 2003; Ward et al. 2003). The functional role of this increase in ipsilateral motor cortical activity may differ depending upon whether the lesioned pathway projected to motor neurons controlling the distal, proximal or axial musculature. Studies investigating the control of distal arm muscles have found the presence of ipsilateral activity to be associated with poor motor recovery (Ward et al. 2003; Werhahn et al. 2003). In contrast, studies of axial muscle activity have found a positive correlation between ipsilateral activity and recovery level (Fujiwara et al. 2001; Hamdy and Rothwell 1998; Muellbacher et al. 1999). The disparity in the relationship between ipsilateral activity and motor recovery between muscle groups may be explained by the fact that distal muscles are primarily innervated by contralateral corticospinal projections (Palmer and Ashby 1992), whereas axial muscles receive extensive bilateral input from cortico-bulbospinal pathways (Ferbert et al. 1992; Hamdy and Rothwell 1998). Therefore, even when contralateral projections are damaged, the axial muscles may be able to depend on a strong ipsilateral projection to a much greater extent than distal muscles.
Currently, the relationship between ipsilateral activity and motor recovery of the proximal upper limb muscles is unknown. After a hemiparetic stroke, the proximal limb is less affected than the distal limb (Colebatch and Gandevia 1989), nevertheless, normal function of the proximal upper arm is frequently severely impaired. Proximal upper limb impairment is characterized by weakness (Adams et al. 1990; Bohannon and Smith 1987; Colebatch and Gandevia 1989; Lum et al. 2003; Mercier and Bourbonnais 2004) and abnormal coordination between elbow and shoulder muscles expressed as flexor and extensor synergies (Twitchell 1951; Brunnstrom 1970; Beer et al. 1999; Dewald and Beer 2001; Dewald et al. 1995; Ellis et al. 2007). These impairments significantly affect reaching abilities in stroke survivors with poor recovery (Beer et al. 2004, 2007; Sukal et al. 2007).
In able-bodied individuals, ipsilateral projections to the proximal upper limb are more common than in the distal upper limb. Studies using TMS have shown that ipsilateral motor evoked potentials (ipsilateral MEPs) in the pectoralis major and the trapezius muscles could be readily evoked during low level tonic contractions, whereas ipsilateral MEPs in the deltoids and biceps occurred only with biphasic contractions (Bawa et al. 2004) and ipsilateral MEPs in distal muscles were rare. Ipsilateral MEPs have been reported in a subset of distal upper limb muscles (finger and wrist extensors, but not the flexors), but only using high stimulation intensities and background contraction (Ziemann et al. 1999). A variety of pathways could mediate the proximal and axial ipsilateral MEPs, including ipsilateral corticospinal, transcallosal or cortico-bulbospinal pathways. In particular, ipsilateral corticospinal (Brinkman and Kuypers 1973) and cortico-bulbospinal pathways preferentially project to medial motor nuclei of the anterior horn and thus are considered to be important in the control of axial and proximal limb muscles (Kuypers 1964).
The anatomical arrangements of ipsilateral projections to proximal muscles may contribute to the relative preservation of proximal limb control and facilitate recovery following stroke. In spite of this, the loss of contralateral corticospinal input to the proximal limb, and subsequent expression of, or take-over by, ipsilateral pathways may explain the loss of independent joint control and abnormal coordination observed in the proximal limb after stroke. In moderate to severely impaired stroke survivors, this abnormal coordination is characterized by an obligatory co-activation between shoulder adductors and elbow extensors (extensor synergy) as well as between shoulder abductors and elbow flexors (flexor synergy) (Brunnstrom 1970; Dewald et al. 1995; Dewald and Beer 2001; Ellis et al. 2007).
The purpose of this study was to determine if the relative presence and magnitude of the ipsilateral and contralateral projections to the proximal muscle PMJ were correlated with relative strength, abnormal coordination, and/or Fugl-Meyer score in mild to severely impaired stroke subjects. We studied the PMJ muscle as it is involved in shoulder adduction and internal rotation, which are part of the abnormal extensor synergy that emerges following stroke.
Materials and methods
Subjects
Ten male subjects with left hemiparesis volunteered to participate in the study. All subjects were recruited from the stroke database at the Sensory Motor Performance Program within the Rehabilitation Institute of Chicago. The experiments were performed with informed consent and approval of the local ethics committee. Subjects were evaluated using the upper extremity motor portion of the Fugl-Meyer Motor Assessment (FMA) (Fugl-Meyer et al. 1975) and the Chedoke-McMaster Stroke Assessment (CMSA) (Gowland et al. 1993) prior to the experiment (see Table 1). Lesion location was identified by a neuroradiologist from an anatomical MRI if it was available.
Table 1.
Clinical data for hemiparetic stroke participants
| Participant | Age (years) | Lesion location | FMAa | CMSAb | Impairment group |
|---|---|---|---|---|---|
| S1 | 51 | N/A | 14 | 2 | MS |
| S2 | 58 | N/A | 22 | 2 | MS |
| S3 | 56 | N/A | 23 | 3 | MS |
| S4 | 57 | Thalamus, posterior putamen, posterior limb of internal capsule | 24 | 3 | MS |
| S5 | 48 | Insula, thalamus, basal ganglia, internal capsule | 26 | 3 | MS |
| S6 | 57 | Lateral/posterior frontal lobe, caudate, basal ganglia, internal capsule | 43 | 6 | MM |
| S7 | 66 | Occipital lobe, thalamus, basal ganglia | 49 | 5 | MM |
| S8 | 38 | Temporal lobe, internal capsule | 50 | 7 | MM |
| S9 | 69 | Frontal–parietal cortex and coronal radiata, internal capsule | 51 | 3 | MM |
| S10 | 65 | N/A | 58 | 7 | MM |
MS moderate-to-severe impairment, MM mild-to-moderate impairment
Based on Fugl-Meyer Motor Assessment (FMA) scale (maximum score = 66)
Based on the Chedoke-McMaster Stroke Assessment (CMSA) scale (maximum score = 7)
Experimental set-up
Subjects were secured in a Biodex experimental chair (Biodex Medical systems, Shirley, NY, USA) using waist and shoulder belts to restrict trunk movement. Both the paretic and non-paretic arms were held in the following arm configuration: 90° elbow angle, 55° shoulder abduction angle, and 20° shoulder flexion angle. A fiberglass cast was put on the hand, wrist and forearm of the paretic arm, which was fixed at the wrist to a 6 degree of freedom load cell (Model 45E15A; JR3, Inc., Woodland, CA, USA) to allow for simultaneous measurement of adduction torque at the shoulder and flexion/extension torque at the elbow. The non-paretic arm was strapped to a single degree of freedom load cell (Model FT04433; ATI Industrial Automation, Garner, NC, USA) under the elbow center of rotation for measurement of the shoulder adduction torque. Forces and moments measured by the 6 degree of freedom load cell were converted on-line to torques at the elbow and shoulder of the paretic limb (Beer 1999). Maximum voluntary shoulder adduction was measured in both the paretic and non-paretic limbs in order to set the background contraction required during TMS. In addition, maximum voluntary elbow flexion/extension torques were measured with the 6 degree of freedom load cell from the paretic arm with the purpose of determining the degree of abnormal coupling between shoulder adduction and elbow extensor coordination in the paretic limb. This was realized by normalizing the amount of secondary elbow flexion or extension torque generated during the shoulder adduction maximal contraction with the respective maximum voluntary elbow flexion or extension torque. Real-time visual feedback of the downward force produced at each elbow during shoulder adduction was provided to the subject on a computer monitor. During the experiment, the subject was asked to simultaneously adduct both arms to a level of 5–10% of the voluntary max of the non-paretic limb. With the exception of subject S2, all subjects performed the task with relative ease. Subject S2 had considerably more weakness relative to the non-paretic side and judged the task to be quite difficult but was able to hold the adduction torque in the target range for the full 30 s. Surface electromyographic (EMG) signals were recorded from the sternal head of the right and left pectoralis major (PMJ) muscles. Bipolar active electrodes (Delsys, Boston, MA, USA), with an inter-electrode distance of 1 cm were placed over the muscle belly. The EMG signals were sampled at 2500 Hz, amplified with a gain of 1000 and band-pass filtered at 6–450 Hz.
Transcranial magnetic stimulation
Single pulse TMS at 90% maximal stimulator output (MSO) was applied over an eighteen-site, 2 cm spaced stimulation grid on the scalp surface (nine sites over each hemisphere) with a 70 mm figure-of-eight coil (Magstim Co., UK). The stimulation intensity of 90% MSO was selected based upon a compromise between an intensity high enough to evoke ipsilateral MEPs [normally 50–60% MSO in non-impaired individuals (MacKinnon et al. 2004)], tolerance to stimulation during mapping, and overheating of the coil. We initially completed mapping at all stimulation sites at 90%. The stimulation grid was comprised of two 3 × 3 grids, each centered on a site located 4 cm lateral to the vertex of the head. This site has previously been found to be the optimal site for activation of the ipsilateral PMJ in able-bodied individuals (MacKinnon et al. 2004). TMS was applied over the entire grid in order to account for any shift in the location of the optimal stimulation site that may have occurred in the lesioned hemisphere with cortical reorganization after the stroke. In addition, the investigation of a grid would be able to detect if the ipsilateral hotspot was in a position distinct from the contralateral hotspot as has been previously reported (Chen et al. 2003; Nirkko et al. 2001; Wassermann et al. 1994; Ziemann 1999). If no contralateral MEP was evoked at 90% MSO we normally explored the grid with a higher intensity to see if a MEP could be evoked. The coil was held with the handle straight backward and was oriented to produce currents in a posterior-to-anterior direction. During the experiment, the subject was asked to maintain the target level of adduction force in both arms for thirty seconds while five TMS stimuli were applied. The experimenter verified that both arms were adducting within the target range before stimulating the subject. Two trials of five stimuli were recorded for each stimulation site for a total of ten stimuli per site.
Data analysis
Data were analyzed off-line using custom software developed in Matlab (Mathworks, Inc., Natick, MA, USA). For each stimulation site, the ten trials of unrectified EMGs were ensemble averaged. The size of the motor evoked potential (MEP) was calculated by measuring the peak-to-peak magnitude of the ensemble-averaged signal. The presence of a MEP was determined by evaluating the ensemble averaged signal as well as an overlay of all ten trials. At least five of the ten trials needed to deviate in the same direction from baseline at a similar time-point for the determination that a MEP occurred. The site with the largest peak-to-peak response was defined as the hotspot. Four hotspots were found; the contralateral and ipsilateral hotspots for the non-paretic arm and the contralateral and ipsilateral hotspots for the paretic arm. The signals elicited from these four sites were further analyzed and compared across subjects. The statistical analysis was performed using Data Desk (version 6.1, Data Description, Inc., Ithaca, NY, USA) or STATPAC for Windows. A significance level of 0.05 was used for all the statistical tests.
MEP prevalence
The prevalence of ipsilateral MEPs and contralateral MEPs were compared between the paretic and non-paretic limbs using the Fisher’s Exact test which is suited for small sample sizes.
Relative size of responses
The MEP magnitude was normalized to the maximum voluntary contraction. The peak-to-peak magnitude of the contralateral MEPs and ipsilateral MEPs in the paretic or non-paretic limb were compared using paired t tests.
Laterality
A laterality index (LI) was calculated for both the paretic and non-paretic PMJ for each participant to determine the relative magnitude of the contralateral (cMEP) and ipsilateral (iMEP) MEPs as follows:
where an LI = 1 signifies that only a cMEP was recorded in the muscle and an LI of −1 indicates that only an ipsilateral MEP was elicited. The correlation between the laterality index and the Fugl-Meyer score was tested using the non-parametric Spearman’s correlation coefficient.
Relative strength
The maximum voluntary shoulder adduction torque was recorded in the paretic limb and normalized to the non-paretic limb maximum torque as an indicator of the level of recovery of strength. The correlation between relative strength and laterality index was tested using the parametric Pearson’s correlation coefficient.
Secondary elbow torque
In abnormal coordination after stroke, shoulder adduction is abnormally combined with elbow extension (Dewald and Beer 2001). In this experiment, the secondary torque at the elbow was measured while subjects performed maximal voluntary shoulder adduction. The secondary elbow torque was normalized to the subject’s maximal elbow flexion or extension torque depending on the direction of the secondary torque (+100% indicates that the secondary elbow torque was flexion and was the same size as the maximum elbow flexion and −100% similarly represents extension). The parametric Pearson’s correlation coefficient was used to test the relationship between the laterality index and the normalized secondary torque across subjects. The non-parametric Spearman’s correlation coefficient was calculated to test the correlation between the degree of elbow co-activation and the Fugl-Meyer score.
Spatial analysis
Scalp surface maps were constructed from the average MEP amplitudes for each subject. The center of gravity (CoG) of these maps was calculated based upon the amplitude-weighted mean location over each hemisphere. Paired t-tests were used to test for differences in the anterior–posterior and medial–lateral locations of the CoGs between contralateral and ipsilateral projections on the same hemisphere.
Latency analysis
MEP onset latencies were marked manually by visual inspection on a trial-by-trial basis based upon a clear deflection of the MEP waveform above baseline. Since MEPs were frequently absent or the onset was difficult to judge in a subset of trials, average latencies within subjects were derived from a minimum of five of the ten TMS trials with a clear MEP. An ANOVA was used to test for the effect of arm (paretic vs. non-paretic) and projection type (contralateral vs. ipsilateral). Scheffe posthoc tests were used to determine significant differences between groups in terms of the onset latency.
Results
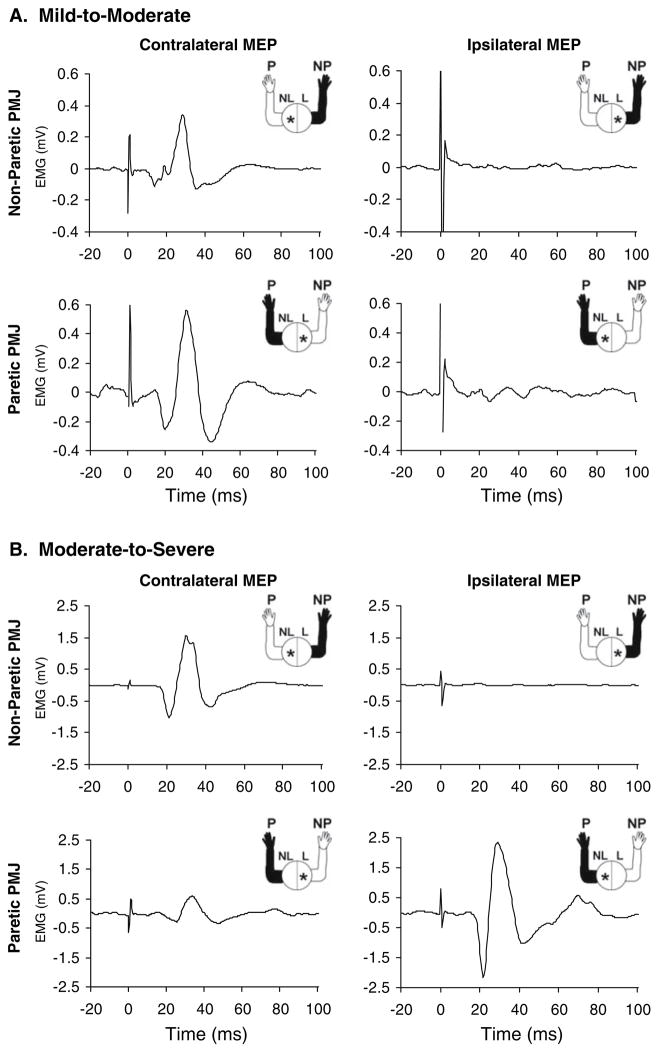
An example of MEPs evoked in the non-paretic and paretic PMJ from either the contralateral or ipsilateral hemispheres is shown in Fig. 1 for two subjects, one subject with mild-to-moderate impairment and one subject with moderate-to-severe impairment. Both subjects had an ipsilateral response in the paretic limb, however, the response was much larger in the moderate-to-severe subject. In fact, the ipsilateral response in this subject was larger than the contralateral response in the paretic limb. We observed ipsilateral responses much more often in the more severely impaired stroke survivors (see Table 2). Based on this observation, we divided the subjects into a mild-to-moderate (MM) impairment group (five subjects) and a moderate-to-severe (MS) impairment group (five subjects). The subjects in the MM group had Fugl-Meyer scores in the 34–66 range and the subjects in the MS group had Fugl-Meyer scores of 1–33 range. All subjects in the MS group had Chedoke–McMaster scores of 3 or less. In contrast, 4 of the 5 MM subjects had Chedoke–McMaster scores of 5 or greater (see Table 1).
Fig. 1.
Motor evoked potentials (MEPs) elicited in the non-paretic (NP) and paretic (P) pectoralis major muscle (PMJ) by transcranial magnetic stimulation of the contralateral and ipsilateral hotspots in two subjects: a a mild-to-moderately (MM) impaired subject, b a moderate-to-severely (MS) impaired subject. Plots show the average MEP waveform from ten trials. The figure in the upper right of each plot shows the arm from which the MEP was recorded (shaded in black) and the hemisphere that was stimulated (asterisk)
Table 2.
Summary of results
| Subjectsa | Group | Non-paretic cMEP (mV) | Non-paretic iMEP (mV) | Paretic cMEP (mV) | Paretic iMEP (mV) | Strengthb (%) | Secondary torquec (%) | Paretic LI | Non-Paretic LI |
|---|---|---|---|---|---|---|---|---|---|
| S1 | MS | 2.59 | 0.08 | 0.93 | 4.50 | 33.18 | −67.70 | −0.66 | 0.94 |
| S2 | MS | 1.46 | 0.09 | x | 0.18 | 5.63 | −162.79 | −1.00 | 0.88 |
| S3 | MS | 1.62 | 0.07 | 0.17 | 0.36 | 57.48 | −74.57 | −0.35 | 0.92 |
| S4 | MS | 1.38 | 0.20 | 0.33 | 0.22 | 56.13 | +31.92 | 0.19 | 0.75 |
| S5 | MS | 0.34 | x | x | 0.05 | 57.19 | −81.20 | −1.00 | 1.00 |
| S6 | MM | 8.28 | 3.21 | 6.84 | 7.08 | 53.04 | −28.82 | −0.02 | 0.44 |
| S7 | MM | 0.03 | x | x | x | 59.03 | +38.93 | N/A | 1.00 |
| S8 | MM | 0.45 | x | 0.08 | 0.10 | 90.06 | +18.41 | −0.11 | 1.00 |
| S9 | MM | 0.47 | x | 0.91 | x | 55.93 | −42.38 | 1.00 | 1.00 |
| S10 | MM | 0.22 | x | 0.21 | x | 57.81 | +1.30 | 1.00 | 1.00 |
x indicates the absence of a MEP, LI laterality index, cMEP contralateral MEP, iMEP ipsilateral MEP
Subjects are listed in order of impairment level from most impaired at the top to least impaired at the bottom
Maximal adduction of the paretic arm normalized to the non-paretic arm
Percentage elbow torque generated during background adduction normalized to maximal elbow flexion or extension respectively (+ signifies flexion, − signifies extension)
Prevalence of contralateral MEPs and ipsilateral MEPs
Contralateral MEPs were evoked in the non-paretic PMJ in all ten subjects (Table 2). In the paretic arm, contralateral MEPs occurred almost as frequently as in the non-paretic PMJ (7/10 vs. 10/10, respectively; P = 0.2105; two-tailed Fisher’s Exact Test, FET). The prevalence of contralateral MEPs was similar between the MS and MM groups for both the paretic and non-paretic limbs. Ipsilateral MEPs in the paretic arm occurred with a similar frequency as in the non-paretic PMJ (7/10 vs. 5/10, respectively; P = 0.6499; two-tailed FET). By impairment group, the subjects in the MS group were more likely to exhibit ipsilateral MEPs in the paretic limb than the MM group (5/5 vs. 2/5, respectively; P = 0.0833; one-tailed FET) and were overall significantly more likely to have ipsilateral MEPs in either limb (9/10 vs. 3/10, respectively; P = 0.0099; two-tailed FET). In only four subjects could all four types of responses be evoked, with three of the four being in the MS group and the fourth having the lowest FM score of the MM group.
Laterality
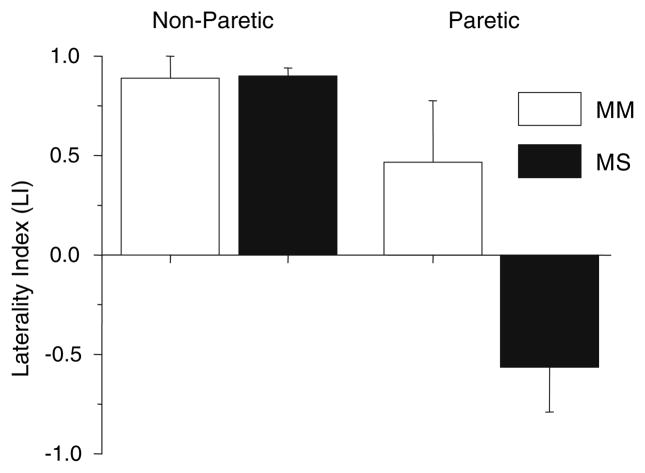
Comparison of the laterality index between the stroke MM and MS groups in the paretic and non-paretic arms was completed using a two-factor (group and arm) mixed design analysis of variance (ANOVA) with repeated measures on the last factor (arm) (Fig. 2). Results show an effect of group (F = 7.58, P < 0.0148), arm (F = 25.876, P < 0.001) and an interaction between group and arm (F = 7.9211, P < 0.0131). The LI in the paretic PMJ of the MS group was significantly different from the non-paretic arm in the same group (P < 0.000226) and the non-paretic (P < 0.000246) and paretic (P < 0.0099) arms in the MM group. There was no significant difference between the other pairs. In one subject (S7) the laterality index could not be determined because no contralateral MEP or ipsilateral MEP was recorded in the paretic limb.
Fig. 2.
Average (±1 standard error) Laterality index (LI) scores for the non-paretic and paretic limbs in the groups with mild-to-moderate (MM) and moderate-to-severe (MS) impairment
Correlation of laterality index with impairment
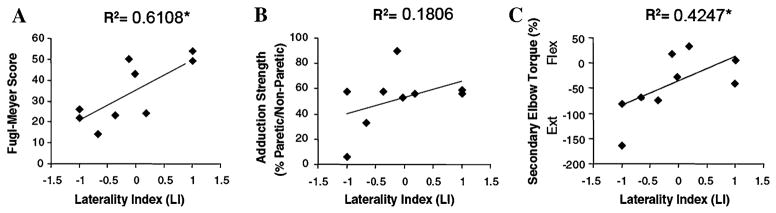
The laterality index in the paretic arm was correlated with impairment level as measured by the Fugl-Meyer score (Spearman’s correlation coefficient = 0.742, P = 0.04) (Fig. 3a). Accordingly, a low Fugl-Meyer score, indicating greater impairment level, was associated with a negative LI, whereas stroke survivors with less impairment (high Fugl-Meyer scores) had a positive LI. Similarly, the amount of elbow flexion/extension torque generated during maximal shoulder adduction, reflecting increased abnormal coordination, was correlated with the LI (Pearson’s correlation coefficient = 0.652, P = 0.05) (Fig. 3c). There was no correlation found between LI and relative strength (Pearson’s correlation coefficient = 0.425, P = 0.25) (Fig. 3b).
Fig. 3.
Correlation between the laterality index (LI) and (a) Fugl-Meyer score, (b) relative strength (percentage of maximum paretic adduction torque relative to the maximum non-paretic adduction torque), and (c) secondary elbow torque (torque generated in the paretic limb during isometric shoulder abduction; normalized to the subject’s maximal elbow flexion or extension torque depending on the direction of the secondary torque; Flex flexion, Ext extension). * Indicates the correlation is significant at the P < 0.05 level
Relative spatial location of optimal contralateral MEPs and ipsilateral MEPs
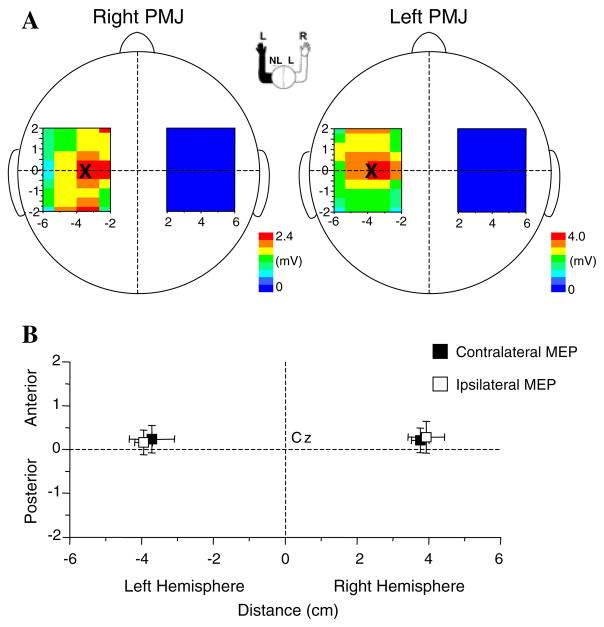
An example of contour maps of the average peak-to-peak MEP amplitudes across the stimulation grid in a single subject is shown in Fig. 4. In this individual, the CoG of the contralateral and ipsilateral projections from the left hemisphere were x, y = −3.5, 0.0 mm and x, y = −3.8, 0.2 mm, respectively. Across subjects, there was no significant difference in the mean locations of the CoG either between or within hemispheres (P > 0.11) (Fig. 4b). The mean locations of the CoG were: left hemisphere, contralateral MEPs, x, y = −3.7, 0.3 mm, ipsilateral MEPs, x, y = −3.8, 0.2 mm; right hemisphere, contralateral MEPs, x, y = 3.8, 0.2 mm, ipsilateral MEPs, x, y = 4.0, 0.3 mm.
Fig. 4.
a Topographic contour maps of the average MEP amplitude evoked by TMS at each of the sites on the grids over the right and left hemisphere of a single subject. The location of the contralateral (figure on the left) and ipsilateral (figure on the right) center of gravity (CoG) on the non-lesioned hemisphere is marked with an “×”. b Plot of the average CoG across subjects for both the ipsilateral and contralateral projections over each hemisphere. NL non-lesioned hemisphere, L lesioned hemisphere, PMJ pectoralis major
Onset latency
In general, the latencies of the contralateral and ipsilateral MEPs evoked in PMJ by TMS of the lesioned hemisphere were delayed relative to the non-lesioned hemisphere. Contralateral and ipsilateral MEPs on the paretic side had average latencies of 20 ± 3 and 24 ± 7 ms, respectively. In contrast, the contralateral and ipsilateral MEP latencies on the non-paretic side were 14 ± 5 and 16 ± 3 ms, respectively. A significant interaction effect was found between the arm involved (non-paretic vs. paretic) and the type of projection (contralateral vs. ipsilateral) (P = 0.0265), but there was no effect of group type (MS vs. MM) (P = 0.3930). A Scheffe post-hoc analysis showed a significant delay in onset for the non-paretic ipsilateral MEP compared to the non-paretic contralateral MEP. Differences between hemispheres in the contralateral and ipsilateral MEP latencies were not significant at the P < 0.05 level.
Discussion
The results of this study show that an increase in the relative magnitude and incidence of an ipsilateral MEP in the pectoralis major muscle, which causes a shift in the laterality index towards the ipsilateral hemisphere, is associated with poor recovery and that the increased expression of ipsilateral projections to the proximal arm muscles may contribute to abnormal intralimb coordination that is observed following stroke.
Ipsilateral MEP prevalence
Ipsilateral MEPs can be elicited in selected arm muscles in non-impaired adults when TMS is applied at high intensity and there is background activation of the target muscle (Carr et al. 1994; Colebatch et al. 1990; MacKinnon et al. 2004; Ziemann et al. 1999). Ipsilateral MEPs are more easily elicited in proximal than distal muscles, however, even when present, the contralateral projections are much stronger (Bawa et al. 2004). In non-impaired individuals, ipsilateral MEPs in the PMJ muscles are typically only about 20% of the contralateral MEP amplitude (MacKinnon et al. 2004), which corresponds to a laterality index score of approximately +0.67. Previous studies have shown that ipsilateral MEPs are elicited more frequently in stroke survivors than in able-bodied individuals (Alagona et al. 2001). In the present study, ipsilateral MEPs were evoked significantly more often in the moderate-severe group than in the mild-moderate group. Moreover, the magnitude of the ipsilateral MEP was frequently larger than the contralateral MEP in the MS group. This resulted in laterality index scores that were significantly lower (more negative) in the MS group paretic limb. In contrast, the average LI scores in the MM group were positive and not significantly different from the non-paretic limb LI.
Both the patient cohort and the task used in this study may have had an influence on the incidence and magnitude of the ipsilateral MEPs observed on the paretic side. All of the subjects tested had a right hemisphere lesion and left hemiparesis. Several TMS studies have shown that the excitability or topographic representation of ipsilateral projections to muscles of the upper limb and trunk in non-impaired individuals are more prominent in the left hemisphere (Ziemann and Hallett 2001; MacKinnon et al. 2004). Ipsilateral activity in the left hemisphere motor areas is particularly enhanced during left limb movements compared to right limb movements, irrespective of handedness (Cramer et al. 1999; Verstynen et al. 2005). Furthermore, it has recently been shown that bilateral movements are associated with a decrease in contralateral and an increase in ipsilateral excitability in both able-bodied individuals and stroke survivors with left hemiparesis (Lewis and Perreault 2007). Taken together, these findings suggest that the large amplitude ipsilateral MEPs we observed in the paretic PMJ may have been influenced by the presence of an intact left hemiphere and the use of a bimanual task.
Evidence for an increased reliance on indirect descending motor pathways following stroke
After stroke, a reduction of the crossed corticospinal input to the muscle could result in an increased dependence on residual brainstem descending pathways (e.g., vestibulo-reticulo- and tectospinal pathways) (Kuypers 1964). These pathways project bilaterally to motoneuron pools of axial and proximal limb muscles and exhibit extensive branching that can innervate neurons over many spinal segments, thus providing the infrastructure for obligatory co-activation of muscles acting at different joints. In particular, reticulospinal pathways may have potent direct input to ipsilateral motoneurons (Jankowska et al. 2003), indirect input to contralateral motoneurons via interneurons and commissural connections, and can influence a variety of muscles in the proximal and distal limb (Davidson and Buford 2004). The fact that the onset latency of ipsilateral MEPs are significantly delayed (typically 5 ms for PMJ) relative to contralateral MEPs suggests these responses are likely mediated by a polysynaptic, possibly corticoreticulospinal, pathway (MacKinnon et al. 2004; Ziemann 1999). This idea is supported by evidence that wrist extensor ipsilateral MEPs can be modulated with neck rotation in accord with the asymmetric tonic neck reflex (Ziemann et al. 1999). Based on this finding, it was suggested that this modulation was mediated by alterations in the excitability of reticulospinal pathways, which receive input from neck receptors. The cortical origin of the pathway that mediates ipsilateral MEPs remains unclear. For movements of the hand, the balance of evidence suggests that ipsilateral activity originates from caudal regions of premotor cortex (area 6) rather than primary motor cortex (Cramer et al. 1999; Ziemann and Hallett 2001; Hanakawa et al. 2005; Verstynen et al. 2005). However, studies of ipsilateral projections to trunk muscles have been equivocal, some showing no difference in the spatial location of the ipsilateral and contralateral CoG (PMJ muscle; MacKinnon et al. 2004) and others showing a medial–posterior shift in the ipsilateral location (deltoid muscle; Wassermann et al. 1994). Thus, it is unclear if ipsilateral MEPs in trunk muscles are mediated by the activation of cortical elements in primary or premotor cortex or both.
In the present study, the onset latencies of the ipsilateral MEPs produced by stimulation of the non-lesioned hemisphere (16 ± 3 ms) were comparable to the ipsilateral MEP latencies reported for PMJ in non-impaired individuals (15 ± 3 ms) (MacKinnon et al. 2004). In contrast, stimulation of the lesioned hemisphere produced contralateral (20 ± 3 ms) and ipsilateral MEPs (24 ± 7 ms) that were delayed relative to projections from the non-lesioned hemisphere. The mean location of the CoG for evoking ipsilateral MEPs on the paretic side (x, y = −3.9, 0.2 mm) corresponded closely to the CoG location in non-impaired individuals (x, y = −4.2, 0.0 mm; MacKinnon et al. 2004). Taken together, these results suggest that the large ipsilateral MEPs were mediated by intact uncrossed projections from the non-lesioned hemisphere to the paretic PMJ. It is also possible that transcallosal pathways contributed to the ipsilateral MEPs observed. Previous studies in non-impaired individuals have been able to discount this possibility due to the absence of responses in patients with agenesis of the corpus callosum or via conduction time arguments (Ziemann et al. 1999). Nonetheless, it is possible that the large ipsilateral MEPs in the stroke participants were mediated by an upregulation of ipsilateral pathways or a convergence of inputs from ipsilateral and transcallosal pathways. The fact that the contralateral MEPs were frequently smaller than the ipsilateral MEPs on the paretic side suggests that a contribution from transcallosal pathways alone would be unlikely to generate the large ipsilateral MEPs observed.
We also found a significant relationship between the LI and the amount of elbow extensor torque generated during shoulder adduction. This abnormal inter-joint coupling is an obligatory co-activation pattern that is observed in moderate-to-severe stroke (Brunnstrom 1970; Dewald and Beer 2001; Dewald et al. 1995, Beer et al. 2007; Ellis et al. 2007, Sukal et al. 2007). There is also abnormal coupling between the shoulder abductors and elbow flexors (flexor synergy) in these stroke survivors. Stimulation of the reticular formation in monkeys has been shown to evoke a pattern of suppression/facilitation of distal and proximal arm muscles consistent with a possible role in the generation of the flexor synergy (Davidson and Buford 2004). Following a stroke, these pathways could be unmasked and produce the flexor and extensor synergies. Thus, the increased expression of ipsilateral MEPs following stroke may be indicative of an increased reliance on corticoreticulospinal pathways and these projections may contribute to the generation of abnormal inter-joint coupling following stroke.
Clinical correlates
In this study, the impairment level, as measured by the Fugl-Meyer index, was correlated with the laterality index such that more severely impaired stroke survivors had greater ipsilateral than contralateral projections to the PMJ. In addition, the laterality index was correlated with the secondary elbow torque during shoulder adduction, reflecting a greater expression of the extensor synergy in subjects with a lower Fugl-Meyer score. This is in agreement with previous studies of distal arm muscles that have shown that the presence of ipsilateral MEPs is correlated with poor recovery (Ward et al. 2003; Werhahn et al. 2003). The loss of independent joint control, demonstrated in this study by the expression of the extensor synergy, could result from either the loss of contralateral projections, or the upregulation of the ipsilateral projection.
While some studies have shown a correlation between the expression of ipsilateral pathways after stroke and poor recovery (Ward et al. 2003; Werhahn et al. 2003), we propose that the upregulation of ipsilateral cortical activity, and increased excitability of ipsilateral corticofugal pathways, may play an important role in preserving some degree of motor function. There is considerable evidence that the ipsilateral hemisphere contributes to the control of normal voluntary movements (e.g., Kristeva et al. 1991; Cramer et al. 1999; Verstynen et al. 2005) and that unilateral lesions following stroke result in deficits in movement control on the ipsilesional side (Wetter et al. 2005; Schaefer et al. 2007). However, utilization of the ipsilateral pathway may come at the expense of independent joint control, which limits the functional usage of the paretic upper extremity. In keeping with this idea, decreased performance during a complex hand movement task has been shown to correlate with increased ipsilateral activity in able-bodied individuals (Verstynen et al. 2005). The present study investigated the PMJ muscle because ipsilateral MEPs in this muscle had been previously recorded in able-bodied individuals and increased the likelihood of eliciting ipsilateral MEPs in stroke. However, PMJ is involved in the arm adduction and internal rotational motion associated with the extensor synergy, which is less strongly expressed following stroke than the flexor synergy. For this reason, the excitability and prevalence of ipsilateral pathways may be even greater in shoulder abductor and elbow flexor muscles involved in the flexor synergy. Nonetheless, this study is the first to demonstrate preliminary evidence of the connection between ipsilateral projections and the expression of abnormal coordination patterns following stroke. Further research investigating the loss of corticospinal contralateral projections and the employment of oligosynaptic ipsilateral projections in relation to specific impairments needs to be undertaken.
Acknowledgments
We thank Dr. Annette Weiss-McNulty for her evaluation of the anatomical MRIs and subject recruitment. This work was supported by grants from the National Institutes of Health (RO1–NS-40902, R01 HD047569-01A1 and 2R01 HD047569-06A1) and the Department of Education (NIDRR - H133G030143).
Contributor Information
Susan Schwerin, Email: sc.schwerin@yahoo.com, Institute for Neuroscience, Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, USA.
Julius P. A. Dewald, Email: j-dewald@northwestern.edu, Institute for Neuroscience, Physical Therapy and Human Movement Sciences, Physical Medicine and Rehabilitation, Biomedical Engineering, Northwestern University, Evanston, USA
Matthew Haztl, Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, USA.
Steven Jovanovich, Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, USA.
Michael Nickeas, Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, USA.
Colum MacKinnon, Email: c-mackinnon@northwestern.edu, Department of Neurology, Institute for Neuroscience, Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, USA.
References
- Adams RW, Gandevia SC, Skuse NF. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain. 1990;113(Pt 5):1459–1476. doi: 10.1093/brain/113.5.1459. [DOI] [PubMed] [Google Scholar]
- Alagona G, Delvaux V, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ, et al. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32:1304–1309. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res. 2004;158:385–390. doi: 10.1007/s00221-004-2031-x. [DOI] [PubMed] [Google Scholar]
- Beer RFDJ. The relationship between abnormal static torque synergies and disturbances of planar arm movements in hemi-paretic subjects: preliminary results. Soc Neurosci Abstr. 1999:25. [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- Beer RF, Ellis MD, Holubar BG, Dewald JPA. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve. 2007;36(2):242–250. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Assessment of strength deficits in eight paretic upper extremity muscle groups of stroke patients with hemiplegia. Phys Ther. 1987;67:522–525. doi: 10.1093/ptj/67.4.522. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain. 1973;96:653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. Medical Dept. Harper & Row; New York: 1970. [Google Scholar]
- Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–122. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol. 1994;475:217–227. doi: 10.1113/jphysiol.1994.sp020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112(Pt 3):749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113(Pt 6):1843–1856. doi: 10.1093/brain/113.6.1843. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81:383–387. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Acosta AM, Yao J, Dewald JPA. Position-dependent torque coupling and associated muscle activation in the hemi-paretic upper extremity. Exp Brain Res. 2007;176(4):594–602. doi: 10.1007/s00221-006-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Caramia D, Priori A, Bertolasi L, Rothwell JC. Cortical projection to erector spinae muscles in man as assessed by focal transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:382–387. doi: 10.1016/0168-5597(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Fujiwara T, Sonoda S, Okajima Y, Chino N. The relationships between trunk function and the findings of transcranial magnetic stimulation among patients with stroke. J Rehabil Med. 2001;33:249–255. doi: 10.1080/165019701753236428. [DOI] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster stroke assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- Green JB, Bialy Y, Sora E, Ricamato A. High-resolution EEG in poststroke hemiparesis can identify ipsilateral generators during motor tasks. Stroke. 1999;30:2659–2665. doi: 10.1161/01.str.30.12.2659. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Parikh S, Bruno MK, Hallett M. Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol. 2005;93:2950–2958. doi: 10.1152/jn.00784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC. Gut feelings about recovery after stroke: the organization and reorganization of human swallowing motor cortex. Trends Neurosci. 1998;21:278–282. doi: 10.1016/s0166-2236(97)01212-5. [DOI] [PubMed] [Google Scholar]
- Honda M, Nagamine T, Fukuyama H, Yonekura Y, Kimura J, Shibasaki H. Movement-related cortical potentials and regional cerebral blood flow change in patients with stroke after motor recovery. J Neurol Sci. 1997;146:117–126. doi: 10.1016/s0022-510x(96)00291-2. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B, Kunkel A, Muhlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10:807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Cheyne D, Deecke L. Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroenceph Clin Neurophysiol. 1991;81:284–298. doi: 10.1016/0168-5597(91)90015-p. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. The descending pathways to the spinal cord, their anatomy and function. Prog Brain Res. 1964;11:178–202. doi: 10.1016/s0079-6123(08)64048-0. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ. Side of lesion influences bilateral activation in chronic, post-stroke hemiparesis. Clin Neurophysiol. 2007;118(9):2050–2062. doi: 10.1016/j.clinph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Quartarone A, Rothwell JC. Inter-hemispheric asymmetry of ipsilateral corticofugal projections to proximal muscles in humans. Exp Brain Res. 2004;157:225–233. doi: 10.1007/s00221-004-1836-y. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Mercier C, Bourbonnais D. Relative shoulder flexor and handgrip strength is related to upper limb function after stroke. Clin Rehabil. 2004;18:215–221. doi: 10.1191/0269215504cr724oa. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Artner C, Mamoli B. The role of the intact hemisphere in recovery of midline muscles after recent mono-hemispheric stroke. J Neurol. 1999;246:250–256. doi: 10.1007/s004150050343. [DOI] [PubMed] [Google Scholar]
- Nirkko AC, Ozdoba C, Redmond SM, Burki M, Schroth G, Hess CW, et al. Different ipsilateral representations for distal and proximal movements in the sensorimotor cortex: activation and deactivation patterns. Neuroimage. 2001;13:825–835. doi: 10.1006/nimg.2000.0739. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol. 2004;190:425–432. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Strens LH, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13:1201–1205. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- Sukal TM, Ellis MD, Dewald JPA. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183(2):215–253. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Verleger R, Adam S, Rose M, Vollmer C, Wauschkuhn B, Kompf D. Control of hand movements after striatocapsular stroke: high-resolution temporal analysis of the function of ipsilateral activation. Clin Neurophysiol. 2003;114:1468–1476. doi: 10.1016/s1388-2457(03)00125-1. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Exp Brain Res. 1994;100:121–132. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–781. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:127–36. [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518(Pt 3):895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Hemispheric asymmetry of ipsilateral motor cortex activation during unilateral motor tasks: further evidence for motor dominance. Clin Neurophys. 2001;112:107–113. doi: 10.1016/s1388-2457(00)00502-2. [DOI] [PubMed] [Google Scholar]