Fig. 3.
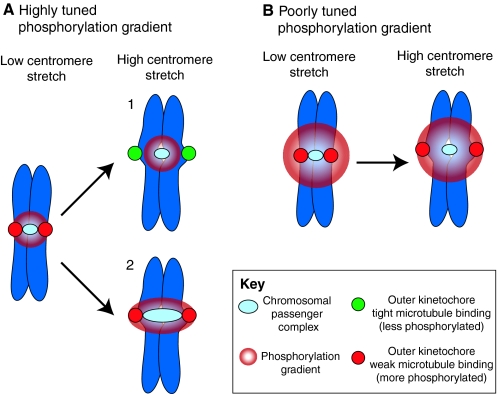
Can kinetochore phosphorylation and microtubule-attachment stability be sufficiently regulated by a phosphorylation gradient and centromere stretch? (A) A highly tuned Aurora B phosphorylation gradient extends from the chromosomal passenger complex (CPC) (light blue), localized to the inner centromere, to just beyond the kinetochores (red) under conditions of low centromere stretch. When centromere stretch is introduced there are two possibilities: (1) the most commonly drawn version of this model suggests that the CPC is maintained in a fixed position upon introduction of centromere stretch extending the kinetochores (now green) beyond the range of the highly tuned phosphorylation gradient. This decreases the likelihood of kinetochore phosphorylation and increases its affinity for kinetochore microtubules. (2) Experimental evidence has shown that the CPC is stretched along with the centromeric chromatin. As the position of the kinetochore (red) has not changed relative to the source of the gradient, centromere stretch cannot move the kinetochores beyond the range of the highly tuned phosphorylation gradient and tight binding cannot be promoted. (B) A poorly tuned phosphorylation gradient extends well beyond the sister kinetochores and introduction of centromere stretch is not sufficient to position kinetochores outside its reach. In this case, it would not matter whether the CPC is stretched, so only one possibility is shown. The only example that provides the spatial resolution required for precise regulation of phosphorylation state and attachment stability is model 1 in A, and experimental evidence suggests that the CPC is not maintained in a fixed position when the centromeric chromatin is stretched. Thus, alternative (related) models should also be considered.