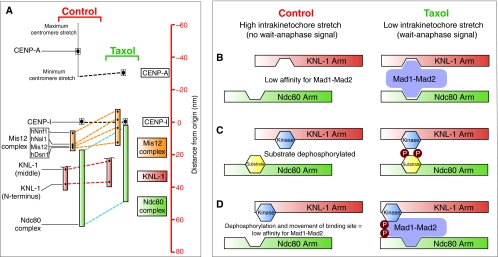
Fig. 6.
Speculative models for translating the relative movement of two mechanical elements within the kinetochore into a wait-anaphase signal. (A)A high-resolution map of the kinetochore reveals two mechanical arms within the kinetochore (KNL-1 arm and Ndc80 arm) that move relative to each other (Wan et al., 2009). Taxol treatment reduces intrakinetochore stretch in part by causing the Ndc80 arm (green) to move inward (represented by blue dashed line) toward the inner kinetochore component CENP-I (set as the origin, distance=0 nm) relative to the KNL-1 arm (green), which maintains a relatively constant distance from CENP-I (red dashed line). The linkage between these two arms is the Mis12 complex (orange) consisting of four components: hNnf1, hNsl1, Mis12 and hDsn1. This complex undergoes a conformational change in the presence of taxol, whereby one end of the complex (hNnf1) shifts inwards while the other end (Dsn1) does not. The C-terminus of KNL-1 is not shown because it was not mapped; however, it has been reported to interact with the Mis12 complex (Kiyomitsu et al., 2007). The CENP-I mark probably represents the periphery of the centromeric chromatin (Wan et al., 2009). (B-D) The geometrical arrangement of the two arms could affect SAC signaling by regulating the localization and/or phosphorylation of checkpoint components. (B) A lock-and-key model for Mad1-Mad2 localization to the kinetochore. Low intrakinetochore stretch promotes Mad1-Mad2 binding at the kinetochore by positioning multiple low-affinity binding sites near each other. The affinity of Mad1-Mad2 is reduced upon introduction of intrakinetochore stretch and the SAC is satisfied. The schematic drawing of Mad1-Mad2 is not meant to convey information about the structural organization of the components but is simply meant to show kinetochore binding sites on the complex that, for example, might only be present in Mad1. (C) Relative positioning of a kinase and its substrate promotes phosphorylation of the substrate under low intrakinetochore stretch and reduced phosphorylation of the substrate upon repositioning of the two components when intrakinetochore stretch increases. (D) A model combining lock-and-key with phospho-regulation. Mad1-Mad2 affinity for the kinetochore is promoted by localized phosphorylation near its binding site or of Mad1-Mad2 itself. The affinity of Mad1-Mad2 for the kinetochore is reduced by increased intrakinetochore stretch because of repositioning of the binding site and dephosphorylation.