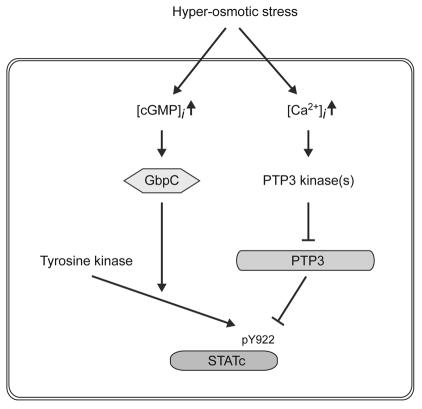
Fig. 4.
Two parallel pathways for activation of STATc by osmotic stress. A semi-constitutively acting tyrosine kinase is proposed to phosphorylate STATc. Hyperosmotic stress is suggested to activate two parallel, oppositely acting pathways: one a cGMP-dependent and Ca2+-independent phosphorylation pathway that upregulates tyrosine kinase activity, the other a cGMP-independent and Ca2+-dependent dephosphorylation pathway that acts directly on STATc. We could not detect significant changes in Ca2+ concentration in the cytoplasm after hyperosmotic stress (our unpublished data), but it is possible that a local Ca2+ increase in a particular part of the cell might have an effect on the phosphorylation of PTP3. Because we are unable to disrupt the PTP3 gene, we cannot rule out other possibilities for the cGMP and GbpC pathway. It could activate other, as-yet-unmapped, phosphorylation sites on PTP3 or it could alter the association of PTP3 with STATc.