Abstract
Repair of DNA damage through homologous recombination (HR) pathways plays a crucial role in maintaining genome stability. However, overstimulation of HR pathways in response to genotoxic stress may abnormally elevate recombination frequencies, leading to increased mutation rates and delayed genomic instability. Radiation-induced genomic instability has been detected after exposure to both low- and high-linear energy transfer (LET) radiations, but the mechanisms responsible for initiating or propagating genomic instability are not known. We have demonstrated that WR-1065, the active metabolite of amifostine, protects against radiation-induced cell killing and delayed genomic instability. We hypothesize that hyperstimulation of HR pathways plays a mechanistic role in radiation-induced genomic instability and that, in part, WR-1065 exerts it radioprotective effect through suppression of the HR pathway. Results of this study demonstrate that WR-1065 treatment selectively protected against radiation-induced cell killing in HR-proficient cell lines compared to an HR-deficient cell line. Further, WR-1065 treatment decreases HR in response to DNA damage using two different mammalian cell systems. This suppression of hyper-recombination is a previously unrecognized mechanism by which WR-1065 effects radioprotection in mammalian cells.
INTRODUCTION
One of the major concerns regarding the use of radioprotective agents is that, while they protect cells from the acute effects of radiation, the surviving cells and their progeny are at an increased risk for delayed radiation-induced genomic instability. Genomic instability is defined as the increased rate of alterations to the genome, including gene mutations, chromosomal abnormalities, micronucleus formation, reduced plating efficiency and cellular transformation. Genomic instability has been detected after both low- and high-linear energy transfer (LET) radiation exposure (1). The severity of the effect depends on a variety of factors including genetics and radiation quality (LET, dose, dose rate). Currently, the mechanisms responsible for initiating and perpetuating delayed genomic instability are unknown; however, several pathways have been suggested (2, 3). We hypothesize that overstimulation of the homologous recombination (HR) DNA double-strand repair pathway leads to hyper-recombination and increased genomic instability in irradiated cells (4).
We developed and characterized a novel green fluorescence protein (GFP) reporter assay for investigating delayed effects of exposure to ionizing radiation as measured by deletion/mutation events and/or homologous recombination in human cells (4). In this model system a recombination or mutation event in single genetically unstable GFP+ or GFP− cells can result in a mixture of green and clear cells within the same colony during clonal expansion (GFP+/−). This experimental system was used to measure delayed genomic instability induced by exposure to low-LET X rays (4), high-LET iron ions (5), or UV radiation (6). Results of these studies demonstrated that WR-1065, the active metabolite of amifostine, decreased genetic instability in the progeny of irradiated cells (5).
Amifostine and its dephosphorylated metabolite WR-1065 can protect against the immediate and delayed effects of radiation exposure. Immediate radioprotective effects of WR-1065 include free radical scavenging, auto-oxidation leading to intracellular hypoxia, and chemical repair by donating hydrogen to radiation-damaged DNA (7, 8). Delayed radioprotective effects of WR-1065 include up-regulation of manganese superoxide dismutase (MnSOD) protein levels and activity, resulting in free radical scavenging (9, 10), and stimulation of endogenous polyamine levels stabilizing chromatin to facilitate DNA repair (11).
However, other WR-1065-mediated mechanisms of protection against genomic instability cannot be ruled out. Pathways other than free radical/reactive oxygen species scavenging could be especially important for protection from high-LET radiations such as those commonly encountered by astronauts in space. DNA damage after high-LET irradiation is caused primarily by direct interactions between high-Z and -energy (HZE) charged particles and DNA, not indirectly through production of hydroxyl free radicals. These differences in radiation quality mean that amifostine/WR-1065 is not as effective in protecting against high-LET radiation-induced cellular damage as it is against low-LET radiation effects. Nonetheless, WR-1065 is still capable of significantly reducing the delayed effects of high-LET radiation exposure, including genomic instability as measured by DNA hyper-recombination/mutation (5).
In this study we investigated the influence of WR-1065 on homologous recombination in mammalian cells and the possible role of HR in the radioprotective activity of WR-1065. Using two different mammalian experimental systems, we demonstrate that WR-1065 treatment decreases the frequency of DNA damage-induced homologous recombination, thereby protecting cells from the potentially negative effects of hyper-recombination.
MATERIALS AND METHODS
Cell Culture
The S31WT clone of human 46BR.1G1 cells harboring SCneo construct and corrected for ligase 1 deficiency was kindly provided by Dr. A. Tomkinson (University of Maryland, Baltimore) and was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 0.2 mg/ml of hygromycin and 0.67 μg/ml puromycin (12). Cells of the MCF10A apparently normal human breast epithelial cell line were maintained in DMEM/F12 medium containing 5% horse serum, 20 ng/ml EGF, 1 μg/ml cholera toxin, 10 μg/ml insulin and 10 μg/ml hydrocortisone (13). The SPD8 cell line is a derivative of the V79 Chinese hamster lung cell line. The AA8 (wild-type), irs1SF (Xrcc3-deficient) and CXR3 (Xrcc3-restored) cell lines are Chinese hamster ovary (CHO) derivatives. All four Chinese hamster cell lines were generously provided by Dr. T. Helleday (University of Oxford, UK) and were maintained in DMEM containing 10% FBS and penicillin-streptomycin (100 μg/ml and 100 U/ml, respectively) (14, 15). For the SPD8 cells, the medium was supplemented with 6-thioguanine (5 μg/ml) to minimize the frequency of spontaneous mutation reversion prior to treatment (16). All cells were kept at 37°C in a 5% CO2/95% air incubator. The cells were routinely tested for mycoplasma (Bionique, Saranac Lake, NY) and showed no evidence of infection.
WR-1065 Treatment
WR-1065 was obtained from Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Institutes of Health (Bethesda, MD). A 1 M stock solution was prepared in sterile phosphate-buffered saline (PBS) immediately before use. For the short-term WR-1065 treatment, cells were exposed to 4 mM final concentration WR-1065 for 30 min, and then the drug-containing medium was replaced with fresh medium immediately before drug, restriction enzyme or radiation treatment. For the low-dose WR-1065 treatment, cells were treated continuously with 40 μM final concentration WR-1065 for 24 h before drug, restriction enzyme or radiation treatment. For each treatment protocol, control samples were treated with drug vehicle for the same duration.
Irradiation
Cells growing in monolayer cultures were exposed to radiation at ambient temperature. Control samples were sham-treated according to the same protocol, without irradiation. X radiation was delivered using a Pantak HF320 X-ray machine (250 kV peak, 13 mA; half-value layer, 1.65 mm copper) at a dose rate of 2.4 Gy/min (Radiation Oncology Research Laboratory, University of Maryland).
Homologous Recombination in SPD8 Hamster Cells
The assay was conducted according to procedures described previously for SPD8 cells (14). Camptothecin (CPT) was prepared as a 20 mM stock solution in dimethyl sulfoxide (DMSO) and stored at −20°C. Hydroxyurea (HU) was dissolved in sterile PBS immediately before use. Briefly, 1 × 106 cells were treated with 100 nM CPT for 1 h or 0.2 mM HU for 24 h, rinsed three times with PBS, and allowed to recover in fresh medium for 24–48 h. Surviving cells were then trypsinized, counted and plated (0.3 × 106 per plate, in triplicate) in hypoxanthine/aminopterin/thymidine (HAT) selective medium. In addition, two dishes were plated to obtain plating efficiency of the drug-treated cells (200 cells/plate). Colonies were fixed and stained after 7–10 days using 0.1% crystal violet in 20% ethanol. All experiments were repeated independently three times.
Homologous Recombination in S31WT Human Cells
To induce HR, S31WT cells (1 × 106) were transiently transfected with the pCMV3xnlsI-SceI expression vector (30 ng) using Lipofectamine2000 as described previously (17). To test for WR-1065 protection against HR, cells were preincubated with WR-1065, 4 mM for 30 min or 40 μM for 24 h prior to transient transfection. At the end of the 6-h transfection, plates were rinsed three times with PBS and allowed to recover for 48 h in fresh DMEM. Surviving cells were then trypsinized, counted and plated (0.3 × 106 per plate, in triplicate) in 1 mg/ml G-418 selective medium. In addition, two dishes were plated to obtain plating efficiency for the transfected cells (200 cells/plate). Cells were allowed to grow for 14–17 days before staining with 0.1% crystal violet in 20% ethanol. All experiments were repeated independently three times.
Reactive Oxygen Species Levels
Changes in cellular reactive oxygen species (ROS) and/or reactive free radical levels for the S31WT cells after transient transfection with the pCMV3xnlsI-SceI expression vector and WR-1065 treatment were measured using the oxidation-specific probe 5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) and flow cytometry techniques as described previously (18). Cells (1 × 106 per ml) were incubated with freshly prepared 5 μM CM-H2DCFDA for 30 min, washed and analyzed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Dead cells were excluded from analysis by propidium iodide staining.
In Vitro Digestion of SCneo Plasmid with I-SceI Restriction Enzyme
The SCneo plasmid (19) (1 μg) was incubated with 1 U of I-SceI (Fermentas) in the manufacturer recommended buffer for 16 h at 37°C. WR-1065 (0.1–10 mM) was added to samples 5 min before the enzyme was added and remained present during incubation. After digestion the plasmid was purified by standard phenol/chloroform extraction and ethanol precipitation. Recovered DNA was resolved on 1% agarose gel, stained with ethidium bromide, photographed and analyzed for topologic form distribution (SC, supercoiled circular; OC, open circular; L, linear).
RNA Interference
The siRNA (siGenome SMARTpool) anti-Rad51, -Rad51C, -Xrcc3 and control siRNA (siGenome Non-Targeting siRNA pool #2) were obtained from Dharmacon (Lake Placid, NY). MCF-10A cells were transfected with 20 nM siRNA using Oligofectamine according to the manufacturer’s instructions. Target protein expression levels were measured 48–72 h after transfection by SDS-PAGE and Western blotting of whole cell extracts (20). The antibodies for Rad51, Rad51C and Xrcc3 were obtained from Upstate Biotechnology (Lake Placid, NY); the antibody for β-actin (mouse monoclonal) and the HRP-conjugated secondary antibodies were from Sigma.
Clonogenic Cell Survival Assay
Cells were treated with WR-1065 or sham-treated and irradiated with X rays as required for each experiment. After irradiation, cells were harvested by trypsinization, resuspended in fresh culture medium, and replated into 100-mm-diameter culture dishes at densities calculated to yield 50–100 cell colonies per dish. After 10–14 days of incubation, colonies were fixed, stained with 0.1% crystal violet in 20% ethanol, and counted (>50 cells/colony). The plating efficiency of controls was calculated as the number of colonies obtained divided by the number of plated cells. The surviving fraction was defined as the number of colonies obtained divided by the number of cells plated multiplied by the plating efficiency.
Cytotoxicity Assay
The cells were seeded in 60-mm-diameter dishes at 100 cells per dish and allowed to attach. Then WR-1065 was added to final concentrations ranging between 0 and 10 mM. For some dishes, the drug-containing medium was replaced with fresh, drug-free medium after 30 min. There were no changes in SPD8 or MCF10A cell morphology or attachment as a result of the 30-min drug treatment or medium replacement. After 7–10 days of incubation, surviving colonies were fixed, stained and counted. Surviving fractions for each drug treatment were calculated as described above, and data points were fitted to modified Hill’s logistic equation to calculate EC50 concentrations (21).
Statistical Analysis
Means and standard errors were calculated for all data from two to three independent experiments. The means were compared between WR-1065-treated and untreated samples by one-way ANOVA.
RESULTS
WR-1065 Reduces Homologous Recombination Frequencies in SPD8 Cells Treated with HU or CPT
We tested the hypothesis that protection against genomic instability by WR-1065 is related at least in part to its ability to reduce the frequency of HR in response to DNA damage using the SPD8 Chinese hamster cell line (14, 16). In these cells HR between the 5-kb tandem repeats restores the functional HPRT gene that can be selected for in HAT medium (16). HR was induced by treatment with 0.2 mM HU for 24 h or 100 nM CPT for 1 h, and the frequency of recombination events was assessed. These drug concentrations were selected to induce maximal HR at an acceptable level of cytotoxicity. Surviving fractions for 0.2 mM HU and 100 nM CPT were ~60% and 30%, respectively (14, 17). Both HU and CPT treatments increased the HR frequency to 12–15 times the HR frequency of controls (Fig. 1). Preincubation with WR-1065 significantly reduced the HR frequency in both the HU-treated cells and the CPT-treated cells (*P < 0.05 and **P < 0.01, respectively). The reduction in HR frequency was similar using either a low concentration of WR-1065 for 24 h prior to HU or CPT treatment or a high concentration of WR-1065 for 30 min prior to HU or CPT treatment. WR-1065 alone did not interfere with the background frequency of HR.
FIG. 1.
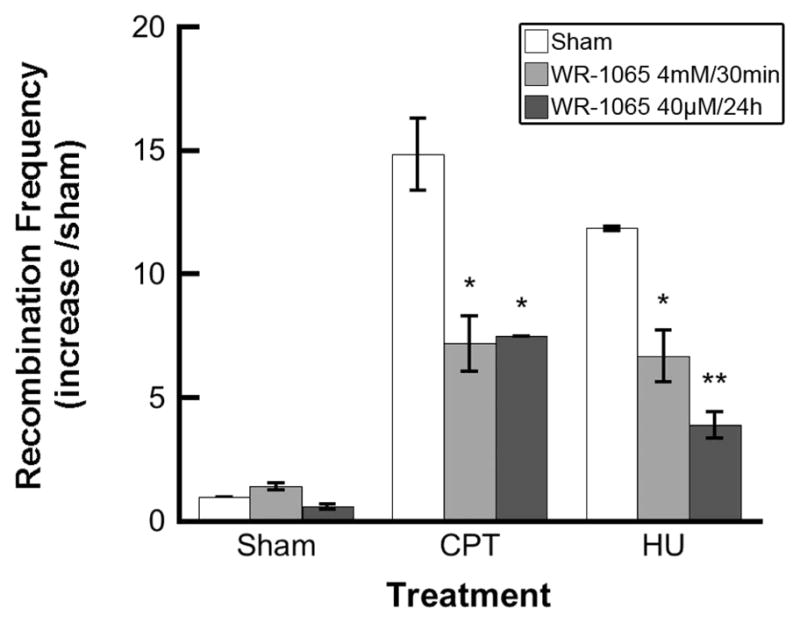
Homologous recombination induced in SPD8 cells by camptothecin (CPT) or hydroxyurea (HU) is reduced by treatment with WR-1065. The cells were incubated with WR-1065 for 30 min at 4 mM (light gray bars) or 24 h at 40 μM (dark gray bars) and then treated with CPT (100 nM for 1 h) or HU (0.2 mM for 24 h) and assayed for homologous recombination frequency as described in the Materials and Methods. Results represent means of three independent experiments ± SEM (*P < 0.05, **P < 0.01).
Cellular Activities of WR-1065 Differ in HR-Proficient and Deficient CHO Cell Lines
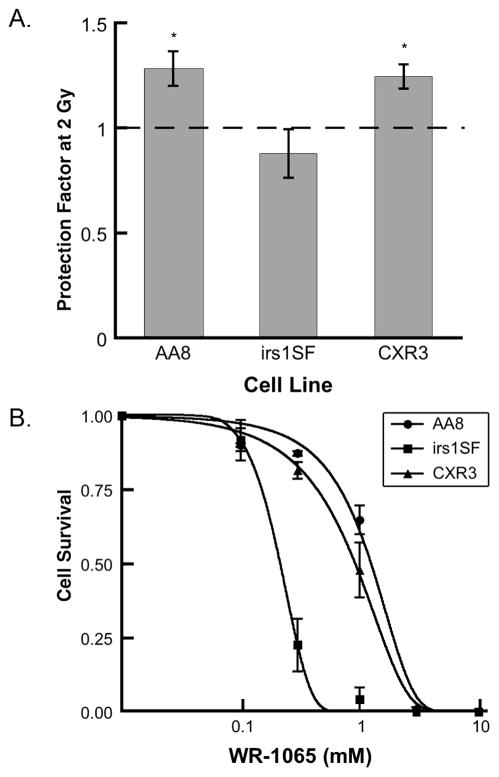
Next we tested whether the radioprotective properties of WR-1065 against cell killing depend on the HR proficiency of the cell line using the 4 mM/30 min WR-1065 treatment protocol (Fig. 2). After irradiation with 2 Gy of X rays, both HR-proficient cell lines (AA8 and CXR3) showed similar levels of protection by WR-1065 against radiation-induced cell killing (P < 0.01). However, the WR-1065-treated and irradiated HR-deficient irs1SF cells exhibited no radioprotection (P > 0.1). The differences between the irs1SF (HR-deficient) and the AA8 and CXR3 cells (both HR-proficient) were statistically significant (Fig. 2; P < 0.05).
FIG. 2.
WR-1065 effects in CHO cells differ between homologous recombination-proficient (AA8 and CXR3) and deficient (irs1SF) cells. Panel A: Radioprotective effects of WR-1065 at 2 Gy. CHO cells were treated with 4 mM WR-1065 for 30 min or sham-treated, then irradiated with 2 Gy and immediately replated for clonogenic survival. Data are normalized to sham-treated controls. Results represent means of five independent experiments ± SEM (*P < 0.05). Panel B: Cytotoxicity of WR-1065. Exponentially growing CHO cells (●, AA8;■, irs1SF; and ▲, CXR3) were treated with WR-1065 (0–10 mM) for 30 min, washed and incubated in drug-free medium for 7 to 10 days. Clonogenic cell survival after drug treatment was measured as described in the Materials and Methods. Results represent means of two independent experiments ± SEM.
To reconcile this observation with the well-documented radioprotective activities of WR-1065, we evaluated the cytotoxicity of WR-1065 in all three CHO cell lines (Fig. 2B). The results of this experiment showed that the HR-deficient irs1SF cell line is approximately five times more sensitive to the cytotoxicity of WR-1065 (EC50 = 0.21 mM) than HR-proficient AA8 and CXR3 lines (EC50 = 1.12 and 0.83 mM, respectively). The decrease in survival for the “protected” and irradiated irs1SF cells relative to the “unprotected” irradiated irs1SF cells is likely a reflection of additive (or synergistic) effects of radiation exposure and WR-1065 cytotoxicity.
WR-1065 Reduces Homologous Recombination Frequencies in SCneo Construct in S31WT Human Cells
We confirmed that WR-1065 treatment reduces the frequency of HR in mammalian cells using an artificial intracellular substrate for HR, SCneo (12, 19). Transient transfection of S31WT cells with the pCMV3xnlsI-SceI vector induced an ~40-fold increase in HR events as a result of I-SceI restriction endonuclease activity compared to sham-transfected controls (Fig. 3). Preincubation with WR-1065 significantly reduced the HR frequency in I-SceI-transfected cells (P < 0.001). Similar to the results obtained using SPD8 cells and HU or CP treatment, WR-1065 protected equally well against restriction endonuclease-induced hyper-recombination using either the 4 mM/30 min or the 40 μM/24 h treatment.
FIG. 3.
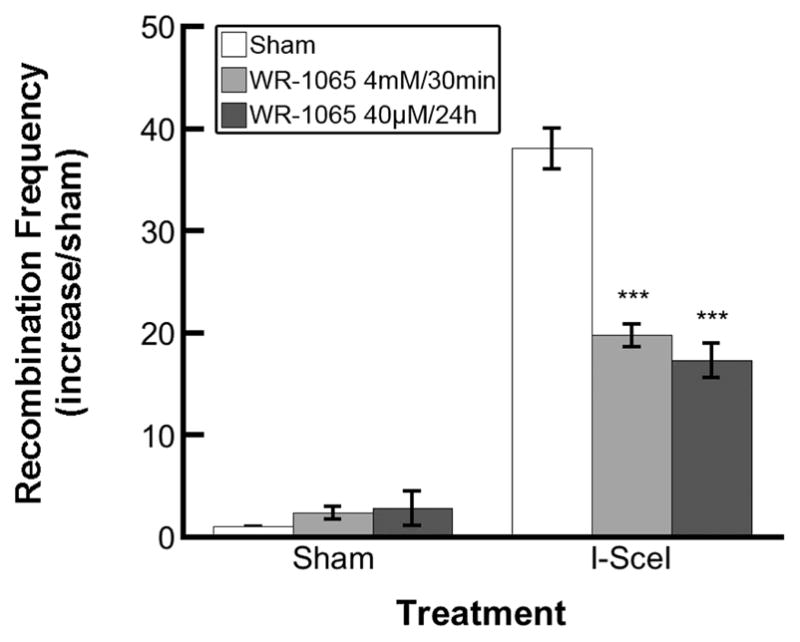
Homologous recombination induced in S31WT cells by I-SceI expression is reduced by treatment with WR-1065. The S31WT cells (harboring SCneo construct) were incubated with WR-1065 for 30 min at 4 mM (light gray bars) or 24 h at 40 μM (dark gray bars), transfected with I-SceI plasmid, and assayed for homologous recombination frequency as described in the Materials and Methods. Results represent means of three independent experiments ± SEM (***P < 0.001).
The SCneo/I-SceI HR experimental system depends on the efficiency of transient transfection as well as on appropriate I-SceI digestion of the recombination substrate. To determine whether WR-1065 pretreatment could interfere with transfection, S31WT cells were transfected with a GFP-expressing plasmid using the same method used for the pCMV3xnlsI-SceI plasmid. Flow cytometry for GFP fluorescence 24 h after transient transfection demonstrated that pretreatment using either WR-1065 protocol had no effect on transfection efficiency (Fig. 4A). In vitro digestion of the SCneo recombination substrate by purified I-SceI enzyme demonstrated that WR-1065 did not interfere with restriction endonuclease activity (Fig. 4B). One unit of I-SceI converts 1 μg of SCneo plasmid from supercoiled (lane 1, SC ~90%) to linear form (lane 2, L), and pretreatment with concentrations of WR-1065 up to 4 mM (lanes 4–7) does not influence the ability of I-SceI to cut SCneo substrate.
FIG. 4.
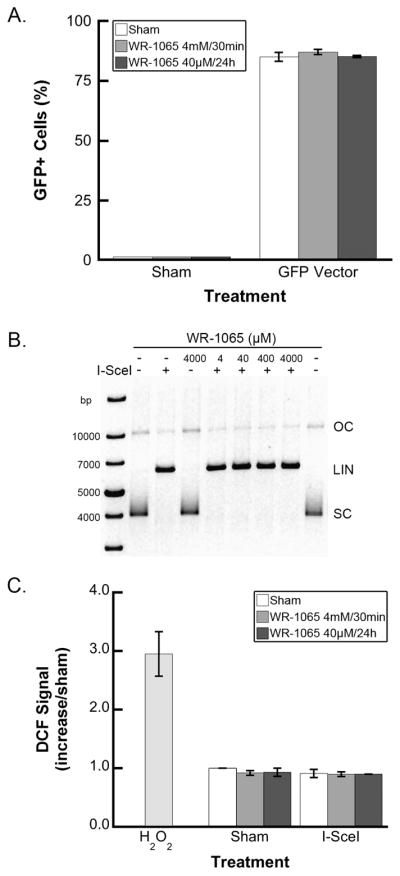
Decrease in homologous recombination frequency after WR-1065 treatment is not caused by WR-1065 preventing DNA transfection (panel A), interfering with I-SceI induction of double-strand breaks in substrate DNA (panel B), or changes in reactive oxygen species levels (panel C). Panel A: S31WT cells were treated with WR-1065 for 30 min at 4 mM (light gray bars) or 24 h at 40 μM (dark gray bars) or were sham-treated with PBS (white bars) and were transfected with GFP plasmid as outlined in the Materials and Methods for the I-SceI transient transfection. After 24 h recovery, the GFP-positive cells were counted by flow cytometry. Results of two independent experiments (±SEM) are shown. Panel B: SCneo supercoiled plasmid (1 μg, lanes 2 and 9, untreated control) was preincubated with WR-1065 (4 μM, lane 5; 40 μM, lane 6; 400 μM, lane 7; 4 mM, lanes 4 and 8) for 5 min followed by 16 h incubation with I-SceI enzyme (1 U, lanes 3 and 5–8). The digestion products were resolved using agarose gel electrophoresis (SC, supercoiled circular; OC, open circular; LIN, linear). Panel C: Changes in cellular reactive oxygen species (ROS) levels after WR-1065 treatment (at 4 mM for 30 min, light gray bars; 40 μM for 24 h, dark gray bars; or sham-treated, white bars) and I-SceI transfection were measured using the specific probe CM-H2DCFDA and flow cytometry as described in the Materials and Methods. H2O2-treated cells serve as an experimental positive control. Results represent means of two independent experiments ± SEM.
Since WR-1065 imparts most of its biological activity through free radical scavenging, we tested whether transfection with and expression of I-SceI changes the levels of ROS and/or reactive free radicals in S31WT cells. Using an oxidation-specific fluorescent probe and flow cytometry, we demonstrated that expression of I-SceI does not change the cellular levels of ROS/free radicals compared to sham-treated controls or hydrogen peroxide-treated positive controls (Fig. 4C).
Effects of WR-1065 in MCF-10A Cells Partially Depleted of Rad51, Rad51C or Xrcc3
Finally, we tested whether depletion of crucial HR factors in MCF-10A human cells through siRNA knockdown would change the radioprotection or cytotoxicity produced by WR-1065. Figure 5A shows that siRNA treatment reduced target protein expression by ~90% for Rad51, ~50% for Rad51C, and ~70% for Xrcc3. When MCF-10A cells were irradiated with 6 Gy of X rays, WR-1065 pretreatment (4 mM/30 min) resulted in a two- to threefold increase in clonogenic survival (Fig. 5B). While knockdown of Rad51 or Rad51C did not alter the radioprotective effects of WR-1065, depletion of Xrcc3 resulted in decreased WR-1065-mediated cell survival. However, the differences between different knockdown cells were not as pronounced as in CHO cell lines (Fig. 2) and were not statistically significant. Evaluation of the cytotoxicity of WR-1065 demonstrated similar sensitivities to cell killing by WR-1065 irrespective of the protein that had been depleted (continuous treatment, Fig. 5C; 30 min treatment, data not shown). Unlike the results obtained for the CHO cell lines, MCF-10A cells depleted of Xrcc3 were as sensitive to the cytotoxicity of WR-1065 as those depleted of Rad51 and Rad51C. One possible explanation for this difference could be the partial depletion of Xrcc3 by the siRNA method as opposed to the complete absence of Xrcc3 in irs1SF cells.
FIG. 5.
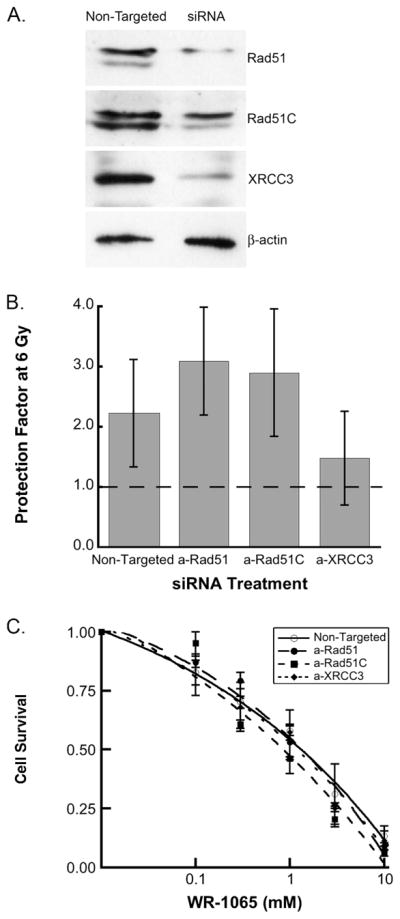
siRNA-directed knockdown of critical factors in homologous recombination (Rad51, Rad51C, Xrcc3) does not significantly change the effects of WR-1065 in human MCF-10A cells. Panel A: Western blot demonstrating knockdown efficiency. Panel B: Radio-protective effects of WR-1065 at 6 Gy. MCF-10A cells were treated with 4 mM WR-1065 for 30 min (gray bars) or sham-treated, irradiated with 6 Gy, and immediately replated for clonogenic survival. Data are normalized to sham-treated controls. Panel C: Cytotoxicity of WR-1065 in MCF-10A cells. siRNA-transfected cells (○, non-targeted siRNA;●, anti-Rad51;■, anti-Rad51C;◆, anti-Xrcc3) were treated continuously with WR-1065 (0–10 mM) for 7 to 10 days. Results represent means of three independent experiments ± SEM.
DISCUSSION
We recently reported that WR-1065 treatment reduces the hyper-recombination/mutation frequency in RKO36 human colon carcinoma cells exposed to ionizing radiation, protecting cells from long-term genomic instability (5). Several mechanisms of the radioprotective activity of WR-1065 have been already proposed. Unfortunately, though, our understanding of WR-1065’s radioprotective activity is far from complete, especially in the case of long-term protection against mutagenesis and genomic instability. In this work we determined that WR-1065 directly influences HR and that HR deficiency changes the radioprotective effects of WR-1065 in mammalian cells.
HR is an essential cellular process required for proper repair of complex DNA damage, especially double-strand damage such as double-strand breaks and interstrand crosslinks. HR also provides support for DNA replication by recovering stalled or broken replication forks (22). However, overstimulation of HR after DNA damage can result in an increased frequency of inappropriate recombination events such as gene conversion and mutations and give rise to genomic instability (23, 24).
In our present work, we observed a significant decrease in HR frequency in mammalian cells pretreated with WR-1065 and challenged with an HR-inducing treatment. Similar responses were registered irrespective of the cell line (both hamster and human) and the method used to induce HR (HU, CPT, restriction enzyme I-SceI). HU inhibits ribonucleotide reductase, depleting cells of several deoxyribonucleoside triphosphates, which in turn stalls replication forks (25). CPT stabilizes topoisomerase I-cleavable complexes, leaving open single-strand breaks that are converted into double-strand breaks during replication, producing collapsed replication forks (26). The I-SceI rare-cutting restriction enzyme produces sequence-specific “clean” double-strand breaks and is used extensively for direct induction of HR in an artificial recombination substrate (19, 27). All three treatments were used previously and showed a high-efficiency stimulation of HR in mammalian cells (17).
It could be argued that the observed reduction in the frequency of HR in cells treated with WR-1065 is due to a decrease in initial DNA damage through well-known activities of WR-1065 such as free radical scavenging or chemical repair of damaged DNA. However, in good agreement with published results on the antimutagenic action of WR-1065, even a low micromolar concentration (40 μM) of the drug was efficient in decreasing recombination frequencies. Such a low drug concentration is known to be ineffective in reducing radiation-related initial DNA damage and preventing cell death (5). We demonstrated that, while 40 μM WR-1065 treatment 24 h prior to irradiation did not protect against the immediate DNA damaging effects of irradiation as measured by micronucleus induction, 4 mM WR-1065 treatment 30 min prior to irradiation did reduce the micronucleus frequency. In the case of the I-SceI/SCno system, our control experiments demonstrated that WR-1065, even at the high concentration of 4 mM, does not interfere with the stable transfection of cells with the SCneo substrate or transient transfection of the same cells with I-SceI. In addition, transfection and generation of DNA strand breaks in the I-SceI/SCneo system did not change free radical levels in cells, providing evidence that WR-1065 activity in this system is independent of its free radical scavenging properties. Based on these observations, it is plausible that WR-1065 can interact directly with HR machinery in mammalian cells. Similar decreases in hyper-recombination were observed in yeast treated with amifostine prior to exposure to the DNA-damaging radiomimetic agent bleomycin (28).
One of the ways that WR-1065 interferes with HR could be through its ability to change chromatin structure. Under physiological conditions, WR-1065 is a divalent cation with its 1,3-diaminepropane moiety similar to spermine and spermidine, facilitating its binding to DNA in a manner analogous to polyamines (29). This WR-1065-DNA interaction can change chromatin structure by distorting DNA supercoiling without breaking the molecule. This could affect the processes of DNA replication and facilitate repair (11). Additionally, treatment with WR-1065 results in a significant increase in intracellular synthesis of polyamines (11). Polyamines (e.g. putrescine, spermidine, spermine) are an essential part of chromatin structure due to their polycationic properties, stabilizing DNA through electrostatic interactions. The elevated levels of polyamines or polyamine-like molecules observed after WR-1065 treatment may explain its antirecombinogenic and antimutagenic properties.
Another possible mechanism for the interference of WR-1065 with HR is its ability to block mammalian cells in the G2/M phase of the cell cycle (30), probably through inhibition of DNA topoisomerase II catalytic activity (31). Topoisomerase II is a ubiquitous enzyme that removes knots and tangles from DNA, and it is an essential factor in most DNA processing pathways, including replication and damage repair. WR-1065 inhibits the catalytic activity of topoisomerase II at concentrations as low as 4 μM (32, 33). Since HR is most active in G2/M, prolonging this phase could provide cells with time to correct inappropriate recombination events, reducing hyper-recombination and mutation frequencies. It is also possible that prolonging the G2 phase of the cell cycle allows additional time for inappropriate de novo HR to occur; however, delayed mutagenesis and genomic instability data suggest that this is not the case (5, 34, 35).
We observed decreased radioprotection and increased cytotoxic activity of WR-1065 in Xrcc3-deficient (HR-deficient) cells. According to current models, the Rad51 family of proteins includes Rad51, Xrcc2, Xrcc3, Rad51B, Rad51C and Rad51D, which have crucial and nonredundant roles in HR repair of DSB inter-strand crosslinks in DNA (36). Rad51 binds to single-stranded DNA to form the Rad51 nucleofilament, the crucial structure in HR, and catalyzes DNA strand exchange. Xrcc3 forms complexes with Rad51C and other paralogs, but not Rad51, and catalyzes the homologous strand pairing (37, 38). However, Rad51C is also implicated in nuclear trafficking of Rad51 after DNA damage (39).
It was demonstrated in earlier studies using E. coli that the radioprotective effects of aminothiols similar to amifostine are practically absent in cells deficient in homologous recombination factors (e.g. RecA−) (40). Similar studies in S. cerevisiae confirmed this observation using HR-deficient rad− yeast mutants. Even more significantly, cysteamine and cysteine did not protect haploid yeast cells from the effects of radiation exposure (41). It was concluded that the radioprotective action of aminothiols may be mediated through a recombination-like mechanism, for which the diploid state and HR proficiency are required. A more recent study (42) demonstrated that the RecN gene product is required for amifostine-mediated DNA protection in E. coli cells. RecN protein is involved in homologous recombination, DSB repair (43), and structural maintenance of E. coli chromosome (44).
In our experiments this phenomenon was especially pronounced in Chinese hamster cells (irs1SF compared to AA8) and less marked in human MCF10A cells knocked down for Xrcc3. SiRNA-directed knockdown of Rad51 and Rad51C did not produce changes in WR-1065-mediated radioprotection or cytotoxicity, possibly indicating a specific role for Xrcc3 in the biological activities of WR-1065.
Similar discrepancies between hamster and human cells in responses to WR-1065 were noted previously. For example, non-homologous end joining (NHEJ)-proficient and deficient human cell lines (MO59K and MO59J, respectively) were protected to a similar extent by pretreatment with 4 mM WR-1065 despite their very different base radiosensitivity (45). In contrast, earlier studies using Chinese hamster cells demonstrated that the NHEJ-deficient mutant xrs5 was not protected against the effects of ionizing radiation by WR-1065 (34, 46). This discrepancy was related to differences in NHEJ deficiency (DNA-PKcs−/− compared to Ku−/−) or other factors such as differences in chromatin structures, which could be related to polyamine content. Similar factors could be responsible for differences observed here between human and hamster cell line responses. In addition, the differences between human and hamster cells in the length of cell cycle phases, which translates directly into the proportion of time the cell spends in the HR-proficient S and G2 phases, should be also considered.
In summary, we demonstrated that WR-1065, the active metabolite of amifostine, disrupts HR in mammalian cells, reducing recombination induced by DNA double-strand breaks and broken replication forks. This ability could be responsible at least in part for the long-term radioprotective effects of WR-1065, preventing hyper-recombination and mutagenesis in irradiated cells. In addition, it could be crucial for radioprotective activity of amifostine/WR-1065 against high-LET space radiation exposures. Our future studies will investigate the molecular mechanism(s) by which WR-1065 interferes with HR and establish the relationship between this activity and the overall biological activity of WR-1065.
Acknowledgments
We would like to thank Dr. Alan Tomkinson, University of Maryland, Baltimore for providing S31WT cell line and helpful discussion, Dr. Thomas Helleday, University of Oxford, for providing the Chinese hamster cell lines SPD8, AA8, irs1SF and CXR3 and James Corcoran, University of Maryland, Baltimore, for assistance in manuscript preparation. WR-1065 was provided by Drug Synthesis and Chemistry Branch, National Cancer Institute. This work was supported by NASA grants NNJ05HE73G (WFM/JEB) and NNX07AT42G (JEB), DOE Low Dose grant DE-SC0001271 (DJG), and NIH R01-CA132998 (DJG).
References
- 1.Limoli CL, Ponnaiya B, Corcoran JJ, Giedzinski E, Kaplan MI, Hartmann A, Morgan WF. Genomic instability induced by high and low LET ionizing radiation. Adv Space Res. 2000;25:2107–2117. doi: 10.1016/s0273-1177(99)01062-5. [DOI] [PubMed] [Google Scholar]
- 2.Hall EJ, Hei TK. Genomic instability and bystander effects induced by high-LET radiation. Oncogene. 2003;22:7034–7042. doi: 10.1038/sj.onc.1206900. [DOI] [PubMed] [Google Scholar]
- 3.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Grim S, Smith LE, Kim PM, Nickoloff JA, Goloubeva OG, Morgan WF. Ionizing radiation induces delayed hyperrecombination in mammalian cells. Mol Cell Biol. 2004;24:5060–5068. doi: 10.1128/MCB.24.11.5060-5068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziegielewski J, Baulch JE, Goetz W, Coleman MC, Spitz DR, Murley JS, Grdina DJ, Morgan WF. WR-1065, the active metabolite of amifostine, mitigates radiation-induced delayed genomic instability. Free Radic Biol Med. 2008;45:1674–1681. doi: 10.1016/j.freeradbiomed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durant ST, Paffett KS, Shrivastav M, Timmins GS, Morgan WF, Nickoloff JA. UV radiation induces delayed hyperrecombination associated with hypermutation in human cells. Mol Cell Biol. 2006;26:6047–6055. doi: 10.1128/MCB.00444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grdina DJ, Kataoka Y, Murley JS. Amifostine: mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact. 2000;16:237–279. doi: 10.1515/dmdi.2000.16.4.237. [DOI] [PubMed] [Google Scholar]
- 8.van der Vijgh WJ, Peters GJ. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (Ethyol): preclinical aspects. Semin Oncol. 1994;21:2–7. [PubMed] [Google Scholar]
- 9.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res. 2007;167:465–474. doi: 10.1667/RR0758.1. [DOI] [PubMed] [Google Scholar]
- 10.Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughan AT, Grdina DJ, Meechan PJ, Milner AE, Gordon DJ. Conformational changes in chromatin structure induced by the radioprotective aminothiol, WR 1065. Br J Cancer. 1989;60:893–896. doi: 10.1038/bjc.1989.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz JD, Motycka TA, Han M, Jasin M, Tomkinson AE. DNA Repair. Vol. 4. Amst: 2005. Reduced repair of DNA double-strand breaks by homologous recombination in a DNA ligase I-deficient human cell line; pp. 649–654. [DOI] [PubMed] [Google Scholar]
- 13.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 14.Helleday T, Arnaudeau C, Jenssen D. Effects of carcinogenic agents upon different mechanisms for intragenic recombination in mammalian cells. Carcinogenesis. 1998;19:973–978. doi: 10.1093/carcin/19.6.973. [DOI] [PubMed] [Google Scholar]
- 15.Tebbs RS, Zhao Y, Tucker JD, Scheerer JB, Siciliano MJ, Hwang M, Liu N, Legerski RJ, Thompson LH. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc Natl Acad Sci USA. 1995;92:6354–6358. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helleday T, Arnaudeau C, Jenssen D. A partial hprt gene duplication generated by non-homologous recombination in V79 Chinese hamster cells is eliminated by homologous recombination. J Mol Biol. 1998;279:687–694. doi: 10.1006/jmbi.1998.1809. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 18.Kim GJ, Fiskum GM, Morgan WF. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006;66:10377–10383. doi: 10.1158/0008-5472.CAN-05-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RD, Liu N, Jasin M. Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature. 1999;401:397–399. doi: 10.1038/43932. [DOI] [PubMed] [Google Scholar]
- 20.Dziegielewski J, Beerman TA. Cellular responses to the DNA strand-scission enediyne C-1027 can be independent of ATM, ATR, and DNA-PK kinases. J Biol Chem. 2002;277:20549–20554. doi: 10.1074/jbc.M109897200. [DOI] [PubMed] [Google Scholar]
- 21.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O’Neil NJ, Petalcorin MI, Martin JS, Collis SJ, Cantor SB, Boulton SJ. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135:261–271. doi: 10.1016/j.cell.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson LH, Schild D. The contribution of homologous recombination in preserving genome integrity in mammalian cells. Biochimie. 1999;81:87–105. doi: 10.1016/s0300-9084(99)80042-x. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi V, Pontis E, Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem. 1986;261:16037–16042. [PubMed] [Google Scholar]
- 26.Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y. Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol. 2000;20:3977–3987. doi: 10.1128/mcb.20.11.3977-3987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickoloff JA, Brenneman MA. Analysis of recombinational repair of DNA double-strand breaks in mammalian cells with I-SceI nuclease. Methods Mol Biol. 2004;262:35–52. doi: 10.1385/1-59259-761-0:035. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann GR, Quaranta JL, Shorter RA, Littlefield LG. Modulation of bleomycin-induced mitotic recombination in yeast by the aminothiols cysteamine and WR-1065. Mol Gen Genet. 1995;249:366–374. doi: 10.1007/BF00287098. [DOI] [PubMed] [Google Scholar]
- 29.Smoluk GD, Fahey RC, Ward JF. Equilibrium dialysis studies of the binding of radioprotector compounds to DNA. Radiat Res. 1986;107:194–204. [PubMed] [Google Scholar]
- 30.Murley JS, Grdina DJ, Meechan PJ. Accumulation of CHO cells in G2 phase following exposure to WR-1065. Radiat Res. 1991;126:223–228. [PubMed] [Google Scholar]
- 31.Grdina DJ, Constantinou A, Shigematsu N, Murley JS. Inhibition of topoisomerase II alpha activity in CHO K1 cells by 2-[(aminopropyl)amino]ethanethiol (WR-1065) Radiat Res. 1994;138:44–52. [PubMed] [Google Scholar]
- 32.Murley JS, Constantinou A, Kamath NS, Grdina DJ. WR-1065, an active metabolite of the cytoprotector amifostine, affects phosphorylation of topoisomerase II alpha leading to changes in enzyme activity and cell cycle progression in CHO AA8 cells. Cell Prolif. 1997;30:283–294. doi: 10.1046/j.1365-2184.1997.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder RD, Grdina DJ. Further evidence that the radioprotective aminothiol, WR-1065, catalytically inactivates mammalian topoisomerase II. Cancer Res. 2000;60:1186–1188. [PubMed] [Google Scholar]
- 34.Grdina DJ, Nagy B, Meechan PJ. Effect of an aminothiol (WR-1065) on radiation-induced mutagenesis and cytotoxicity in two repair-deficient mammalian cell lines. In: Nygaard OF, Upton AC, editors. Anticarcinogenesis and Radiation Protection. Vol. 2. Plenum Press; New York: 1991. pp. 287–295. [Google Scholar]
- 35.Aydemir N, Sevim N, Celikler S, Vatan O, Bilaloglu VR. Antimutagenicity of amifostine against the anticancer drug fotemustine in the Drosophila somatic mutation and recombination (SMART) test. Mutat Res. 2009;679:1–5. doi: 10.1016/j.mrgentox.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Thacker J. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 2005;219:125–135. doi: 10.1016/j.canlet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Kurumizaka H, Ikawa S, Nakada M, Eda K, Kagawa W, Takata M, Takeda S, Yokoyama S, Shibata T. Homologous-pairing activity of the human DNA-repair proteins Xrcc3.Rad51C. Proc Natl Acad Sci USA. 2001;98:5538–5543. doi: 10.1073/pnas.091603098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiese C, Collins DW, Albala JS, Thompson LH, Kronenberg A, Schild D. Interactions involving the Rad51 paralogs Rad51C and XRCC3 in human cells. Nucleic Acids Res. 2002;30:1001–1008. doi: 10.1093/nar/30.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gildemeister OS, Sage JM, Knight KL. Cellular redistribution of Rad51 in response to DNA damage: A novel role for Rad51C. J Biol Chem. 2009 doi: 10.1074/jbc.M109.024646. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bresler SE, Noskin LA, Stepanova IM, Kuzovleva NA. Mechanism of the radioprotecting action of chemical compounds on Escherichia coli cells. Mol Gen Genet. 1978;163:75–85. doi: 10.1007/BF00268966. [DOI] [PubMed] [Google Scholar]
- 41.Petin VG, Matrenina VL. Radioprotecting action of chemical compounds on gamma-irradiated yeast cells of various genotypes. Mol Gen Genet. 1981;183:152–157. doi: 10.1007/BF00270154. [DOI] [PubMed] [Google Scholar]
- 42.Almeida E, Fuentes JL, Cuetara E, Prieto E, Llagostera M. Amifostine protection against induced DNA damage in gamma-irradiated Escherichia coli cells depend on recN DNA repair gene product activity. Environ Toxicol. 2009 doi: 10.1002/tox.20483. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Kidane D, Sanchez H, Alonso JC, Graumann PL. Visualization of DNA double-strand break repair in live bacteria reveals dynamic recruitment of Bacillus subtilis RecF, RecO and RecN proteins to distinct sites on the nucleoids. Mol Microbiol. 2004;52:1627–1639. doi: 10.1111/j.1365-2958.2004.04102.x. [DOI] [PubMed] [Google Scholar]
- 44.Meddows TR, Savory AP, Grove JI, Moore T, Lloyd RG. RecN protein and transcription factor DksA combine to promote faithful recombinational repair of DNA double-strand breaks. Mol Microbiol. 2005;57:97–110. doi: 10.1111/j.1365-2958.2005.04677.x. [DOI] [PubMed] [Google Scholar]
- 45.Murray D, Rosenberg E, Allalunis-Turner MJ. Protection of human tumor cells of differing radiosensitivity by WR-1065. Radiat Res. 2000;154:159–162. doi: 10.1667/0033-7587(2000)154[0159:pohtco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Meechan PJ, Haraf DJ, Diamond AM, Grdina DJ. Reversion of radiosensitivity in azacytidine-treated XRS5 cells does not result in full radioprotection by WR-1065. Int J Radiat Oncol Biol Phys. 1992;23:999–1002. doi: 10.1016/0360-3016(92)90905-w. [DOI] [PubMed] [Google Scholar]