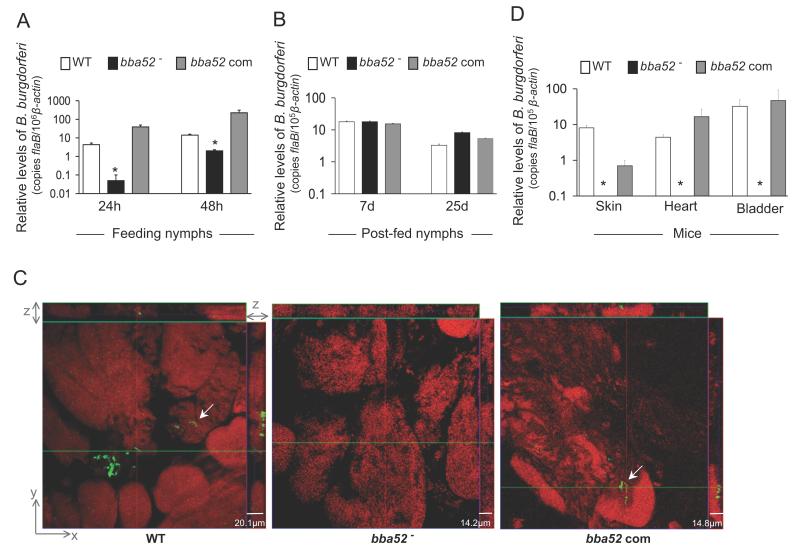
Figure 6. bba52 mutant B. burgdorferi is impaired in its ability to transit between murine hosts and ticks.
(A) B. burgdorferi burdens in ticks during acquisition from infected mice. Mice were infected with B. burgdorferi (3 mice/group) and, following 2 weeks of infection, naïve I. scapularis larvae or nymphs (25 ticks/mouse) were allowed to feed on mice. B. burgdorferi burdens in ticks were analyzed at the indicated time intervals following feeding by measuring copies of the B. burgdorferi flaB RNA. Amounts of tick β-actin were determined in each sample and used to normalize the quantities of spirochete RNA. Bars represent the mean ± SEM of eight qRT-PCR analyses derived from two independent infection experiments. Differences in the spirochete burdens in ticks infected with bba52 mutant and those with the bba52-complemented isolates or wild-type spirochetes were significant both at 24 and (*P < 0.002) 48 hours (*P < 0.02). (B) B. burgdorferi burdens in post-fed ticks. Nymphs were allowed to engorge on infected mice as described in figure 6A and B. burgdorferi burdens in post-fed ticks were analyzed at the indicated time intervals by measuring copies of the B. burgdorferi flaB RNA and normalize against tick β-actin RNA. Bars represent the mean ± SEM of eight qRT-PCR analyses derived from two independent infection experiments. Similar burdens of bba52 mutants, wild type and bba52-complemented isolates were evident at day 7 or day 25 (P > 0.05). (C) B. burgdorferi localization in infected salivary glands during transmission. A representative image showing confocal orthogonal display of infected salivary glands in the XZ and YZ axis revealing the distribution of spirochetes through the full thickness of the 60-hour fed salivary glands is shown. The spirochetes (arrow) were labeled with FITC-labeled goat anti-B. burgdorferi antibody (shown in green) and gland morphology were revealed by labeling of acinar actin filaments using Texas Red-phalloidin (shown in red). While wild type and complemented B. burgdorferi (bba52 Com) were occasionally observed within the gland, bba52 mutant (bba52-) was consistently undetected. (D) B. burgdorferi transmission from infected ticks to mice. B. burgdorferi-infected nymphs were generated by feeding larvae on mice infected with wild type and genetically-manipulated spirochetes, as described in the text. Newly-molted B. burgdorferi-infected nymphs were allowed to feed on naïve mice (1 tick/mouse, 3 animals/group). B. burgdorferi burdens were assessed in the indicated murine tissues after one week of tick feeding by measuring copies of the B. burgdorferi flaB RNA and normalized against mouse β-actin levels. Bars represent the mean ± SEM of four qRT-PCR analyses derived from two independent animal infection experiments. * bba52 mutants were undetectable.