Abstract
This study examines the relation between risk exposures in early life and hazard of mortality among 11,978 Union Army veterans aged 50 and over in 1900. Veterans’ risk exposures prior to enlistment–as approximated by birth season, country of origin, residential region, city size, and height at enlistment–significantly influence their chance of survival after 1900. These effects are robust irrespective of whether or not socioeconomic well-being circa 1900 has been taken into account; however, they are sensitive to the particular age periods that have been selected for survival analysis. Whereas some of the effects such as being born in Ireland and coming from big cities became fully unfolded in the first decade after 1900 and then dissipated over time, the effects of birth season, being born in Germany, residential region in the U.S., and height at enlistment were more salient in the post-1910 periods. Height at enlistment shows a positive association with risk of mortality in the post-1910 periods. Compared to corresponding findings from more recent cohorts, the exceptional rigidity of the effects of risk exposures prior to enlistment on old-age mortality among the veterans highlights the harshness of living conditions early in their life.
INTRODUCTION
Death is an instantaneous event, yet its risk factors can often be examined from a life course perspective. This is especially the case for today’s modern, developed countries where most deaths are caused by degenerative diseases in old age that evolve over a long period of time. Among all deaths in the United States in 1981, the proportion of deaths at age 65 and over was 62 percent (Myers, 1989). Due to the aging of the U.S. population and the compression of mortality into older ages, the corresponding proportion increased to 74 percent in 2002.1 With this trend continuing, it can be expected that old-age mortality will play an increasingly predominant role in determining life span in the future. A successful identification of the life course determinants of old-age mortality and delineation of their pathways is thus crucial for a better understanding of mortality trends in the past and its possible trajectories in the future.
The past two decades have witnessed a growing interest in the relation between early-life conditions and mortality in later life. Accumulative evidence from a number of studies suggests that the nutritional and epidemiological environment circa birth–as approximated by birth weight, birth season and place, and infant mortality rates–can have a long lasting impact on morbidity and mortality in later life (e.g. Barker and Osmond 1992; Barker 1998; Avchen, Scott, and Mason 2001; Doblhammer and Vaupel 2001; Bengtsson and Lindström 2003; Fogel 2004; Costa and Lahey 2005). Childhood conditions such as exposure to diseases, malnutrition, and poverty can all pose a threat to well-being in later life (e.g. Elo and Preston 1992; Davey-Smith, Gunnell, and Ben-Shlomo 2001; Blackwell, Hayward, and Crimmins 2001; Hayward and Gorman 2004; Luo and Waite 2005). Height, as a proxy of net intake of nutrition during growing years (Fogel, Engerman, and Floud 1983; Fogel 1993; Riley 1994), was also found to be related to adulthood mortality (e.g. Waaler 1984; Costa 1993; Fogel and Costa 1997; Fogel 2004).
In light of these empirical findings, two questions are in need of further study. The first pertains to the identification of the age periods in later life when risk exposures in early life can have a significant impact on health and survival, a topic that has received little attention in previous studies. To say that exposures to environmental hazards in early life can negatively influence chance of survival in later life does not necessarily mean that the impact is active across all age periods in later life. This is because the effects of risk exposures in early life can be latent for a long time before their manifestation (Elo and Preston 1992). Moreover, since frailer individuals and individuals who have experienced severe environmental insults early in life are presumably to die earlier, mortality selection can potentially modify or even reverse the presumed relation between early-life conditions and mortality, depending on the specific age period in later life that has been selected for analysis. A better understanding of the relation between risk exposures in early life and mortality in old age thus calls for an assessment of the extent to which the effects of earlier risk exposures can change as a function of particular age periods in later life.
The second pending issue concerns whether and to what extent socioeconomic conditions in later life can modify the observed impact of early-life conditions on survival in later life. Since risk exposures in early life can indirectly influence chance of survival in old age through its effect on adulthood SES, adjusting for the effects of adulthood SES might potentially make a difference in the observed effects of risk exposures in early life. Extant literature has failed to yield a consistent finding on the relative importance of early-life conditions in adulthood mortality. Whereas results from several studies show that adjusting for adulthood SES substantially attenuates or even abolishes the effects of early-life conditions on later mortality (e.g. Ben-Shlomo and Davey-Smith 1991; Hayward and Gorman 2004), findings from some other studies document the significant impacts of early-life conditions on mortality in old age even after SES in later life having been taken into account (e.g. Bengtsson and Lindström 2003; Costa and Lahey 2005).
This study seeks to provide new clues to the above two questions through an analysis of the effects of risk exposures in early life on old-age survival among Union Army veterans who fought the American Civil War in the 1860s. Specifically, the study aims to assess the impacts of risk factors prior to enlistment–as approximated by birth season, birth country, residential region in the U.S., city size, and height at enlistment–on chance of survival after 1900 given that a veteran had survived to 1900 when the average age of the veterans were 61 years old. Information on these variables from the Union Army sample captures some of the variations in the epidemiological environment where the veterans spent their early life, which could potentially explain mortality disparities in their old age.
BACKGROUND
Birth Season and Mortality in Later Life
Birth season can be linked to mortality in later life for at least two reasons. The first lies in the seasonality of the incidence of infectious diseases, which is caused by seasonal changes in pathogen transmission rates (Spira 1981; Koelle, Pascual, and Yunus 2005). As a result, people born in different months or seasons in a year might presumably have differential exposures to pathogens in the beginning of their life. These exposures can potentially pose a lasting impact on health and survival in later life. Certain diseases such as tuberculosis and typhoid, once acquired in early life, could be latent for many years before they manifest their effects in adulthood (Elo and Preston 1992).
The second factor contributing to the association between birth season and mortality in later life is the seasonal variation in food availability. For most of human history, food production and storage followed a rigid seasonal pattern, with a typical abundance of supply during the harvest season and a subsequent paucity during the hunger season. Fresh vegetables and fruits usually become scarce during the spring and winter time. These seasonal changes in the quantity and quality of food supply can significantly alter the level of nutrient intake by pregnant women, which could in turn affect the nutritional status of their newborns (Watson and McDonald 2007). The seasonality of food supply implies that birth season can make a difference in the nutritional wellbeing during the fetal period when the vital organs are still developing. Malnutrition and other insults during this critical period can have an enduring or even lifelong impact on health in later life (Barker and Osmond 1992; Martyn, Barker, and Osmond 1996; Barker 1998).
Several studies have documented the significant role of birth season in mortality disparity at older ages. A comprehensive investigation in this area is a study by Doblhammer and Vaupel (2001) on the relation between month of birth and life expectancy at ages 50 and over. Based on analyses of large sample data on month of birth and mortality from Austria, Denmark, and Australia in the second half of the twentieth century, this study suggests that for people in Austria and Denmark, being born in autumn is associated with lower mortality risk as compared with being born in spring. Consistently, data for Australia show that in the southern hemisphere the pattern is shifted by half a year. Another finding from the study is that mortality selection during infancy does not help explain the observed survival disadvantages associated with being born in spring, as indicated by excess mortality in the first year of life for those who were born in spring. Doblhammer and Vaupel further concluded that remaining life expectancy at age 50 appears to depend on factors that arise in utero or early in infancy, which provides support to the Barker hypothesis. This study also reported that differences in adult lifespan by month of birth decreased over the course of the twentieth century due to substantial improvements in maternal and infant health.
A similar study by Lerchl (2004) examined the relation between month of birth and life expectancy at age 50 and older in Germany. Consistent with what Doblhammer and Vaupel found, Lerchl reported that individuals born in May through July had the lowest age at death, while those born between October and December had the highest. Another finding from this study is that the observed effects of birth season on life expectancy are more pronounced among men than among women.
Country of Origin and Mortality in Later Life
Country of origin can be a valuable indicator of exposures to environmental insults in early life. When individuals from different countries are compared for clues of their differential risk of mortality, the particular country from which they come, in conjunction with relevant historical records of the country, conveys important background information on the epidemiological environment where they spent infancy or childhood such as the climate, food supply, welfare policy, public health infrastructure, incidence and prevalence of diseases, and so forth, all of which could potentially influence chance of survival in later life.
The information on country of origin becomes even more instrumental in capturing risk exposures in early life when subjects’ infancy or childhood synchronizes with the occurrences of well-recorded historical events like famines, wars, or endemics. For instance, millions of Irish fled to the U.S. in the mid nineteenth century due to the Potato Famine, also termed as the Great Famine, which devastated Ireland between 1845 and 1849. For those Irish immigrants who were born or grew up in the famine period, it is very likely that they had suffered from malnutrition during infancy or childhood before their families could manage to migrate to the U.S.
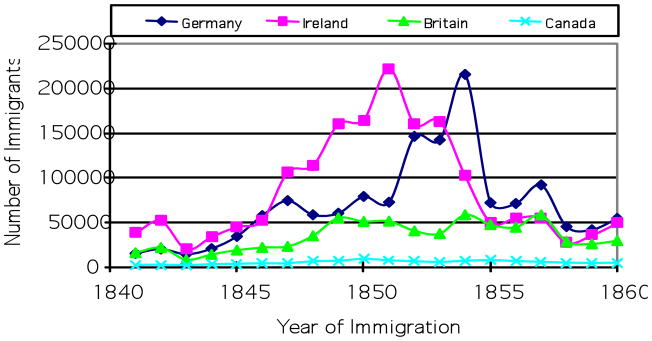
The mid nineteenth century witnessed the beginning of the mass immigration from Europe to the U.S., with most immigrants coming from Ireland, Germany, and Britain. Figure 1 shows the number of immigrants from these three countries and Canada from 1841 to 1860. Between 1847 and 1853, Ireland was the largest sending country of immigration to the U.S., but it was replaced by Germany for the period between 1854 and 1860.
Figure 1.
Immigration to the U.S. by Country of Origin: 1841 to 1860
Source: Historical Statistics of the United States 1789–1945: A Supplemental to the Statistical Abstract of the United States. Bureau of the Census. 1949. Washington, D.C.
The pushing effects of the Potato Famine can be clearly illustrated by the skyrocketing increase in the number of Irish immigrants starting from 1846, with a peak in 1851 when more than 200,000 Irish migrated to the United States in a single year. The harshness of the famine can also be reflected by yearly mortality rates on the voyages from Ireland to New York from 1836 to 1853 as reported by Cohn (1984) who found that the highest mortality rates were observed for the four years from 1846 through 1849.
Relative to native-born Americans, immigrants from Ireland, Germany, and Britain had substantially higher rates of adulthood mortality in the early twentieth century. Based on records of death registration for New York and Pennsylvania in 1910, Dublin and Baker (1920) calculated age-specific death rates for each of the five immigrant groups including British, Germans, Irish, Italians, and Russians, and compared these rates with corresponding rates for native-born Americans of native parentage. The results indicate that for all age groups above 25 and for both males and females, mortality rates for immigrants from Ireland, Germany, and Britain are much higher than those for native-born Americans of native parentage and those for Italian and Russian immigrants. For instance, the death rate for male Irish immigrants at ages between 25 and 44 in 1910 was 18.5 per thousand in New York as compared to 6.9 per thousand for native-born of native parentage in the same age group. The corresponding death rates for Italians and Russians were 6.6 and 5.1 per thousand respectively, suggesting substantial heterogeneity within the immigrant population in terms of chance of survival in adulthood.
The Urban-Rural Distinction in Risk of Mortality
A series of factors are related to the well-documented survival disadvantage associated with living in urban areas in the nineteenth-century U.S. (e.g. Preston and Haines 1991; Haines 2001; Lee 2003; Fogel 2004). First, the higher population density in urban areas means more hosts for communicable diseases and easier transmission of water and airborne diseases. Crowded housing is a major contributing factor to the prevalence of tuberculosis in nineteenth-century Western Europe (Riley 2001). Infectious diseases such as tuberculosis, cholera, typhoid fever, small pox, and malaria caused most of premature deaths in the nineteenth century, which has been well elaborated in the paradigm of the epidemiological transition (Omran 1971).
U.S. cities were poorly equipped to deal with the public health challenges they faced in the nineteenth century. Public health infrastructure such as water treatment and delivery, sewage removal, and housing failed to keep pace with the increase of city population. The delivery of pure water and the removal of waste in big cities in the second half of the nineteenth century required major advances in hydraulic equipment, pipes to deliver the water and carry off the waste, and in new construction techniques required to build tunnels over long distances, which were usually beyond the capacity of the city governments at that time (Fogel 2004).
Moreover, inadequacy in understanding the disease process seriously hampered endeavors by public health agencies to combat communicable diseases in the nineteenth century. Speculations on the linkage between bacteria and infectious diseases began prior to the nineteenth century, but the science of microbiology did not become established until after 1850 (World Health Organization 2003). The lack of knowledge also complicated water delivery in urban areas, which posed a big challenge for public health in the nineteenth century. For instance, in 1897 about half of all American municipalities used lead water pipes (Troesken 2003). But few people at that time were aware of the hazards caused by the use of lead pipes. Troesken’s investigation suggests that in the average town in 1900, the use of lead pipes increased infant mortality and stillbirth rates by 25 to 50 percent.
The booming manufacturing sector in urban areas in the late nineteenth century resulted in more air and water pollution. For example, Wilson (2003) described the trend in the prevalence of chronic respiratory diseases among Union Army veterans. One of the key findings in his study is that the prevalence rate increased between 1895 and 1910. Wilson conjectured that one of the contributing factors could be the deteriorating air quality in urban areas in the period.
Adulthood Height and Mortality
Adulthood height, as a proxy of net intake of nutrition during growing years (Fogel 1993; Riley 1994), has been widely used in longitudinal studies of health disparity. The validity of using adult height as an indicator of exposures to environmental insults during years of growth also lies in the observation that adult height helps capture variations in birth weight. Sørensen et al. (1999) examined the relation between birth weight and adult height among a group of young Danish men. The results reveal a strong positive correlation between birth weight and adult height: for subjects with birth weight less than 2,500 grams the mean height is 175.7 cm, while for those with birth weight more than 4,501 grams the mean height is 184.1 cm. Since lower birth weights are indicative of exposures to malnutrition and other insults during fetal growth, differences in stature can help capture some of the variations in the epidemiological environment in the initial period of life.
DATA AND METHODS
Data: The Union Army Sample
The data used in this study come from the Union Army sample (Fogel 2000, 2001) that contains detailed records on major life events from childhood to death for roughly 36,000 Union Army soldiers who fought the American Civil War. The Union Army sample has three components or sub-samples.
Military records that contain comprehensive demographic information at enlistment such as age, height, city and state of residence, country of origin, occupation and so on; wartime experience such as battles participated in, wounds received, prisoner of war, wartime diseases and so forth; and pension application records for 35,570 veterans from 303 companies that were randomly selected from over 20,000 Union Army recruit companies;
Surgeon’s Certificate Data that incorporate detailed medical records on postwar physical examinations for about 17,700 veterans who claimed to have various wound or disease conditions for pension purposes;
The census data that preserve socioeconomic information for those veterans who can be linked to the U.S. censuses from 1850 to 1910.
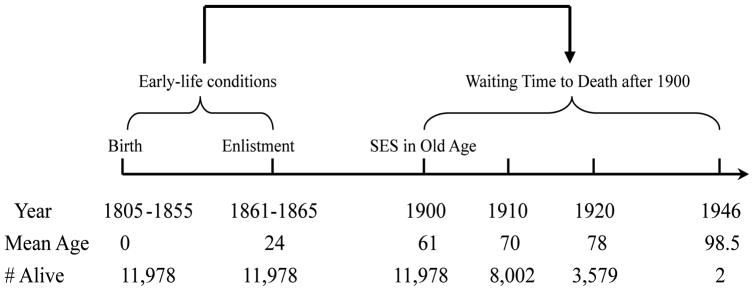
By integrating relevant information from the three sub-samples, I constructed life cycle records for 17,700 veterans in the Surgeon’s Certificate Data covering three life stages including early life, wartime experience, and socioeconomic conditions circa 1900 when the average age of those survived veterans was about 61 years old. Since most of the information on socioeconomic conditions in later life comes from the 1900 census, the working sample for this study includes 11,978 veterans who survived to 1900 and who applied for pensions prior to 1900. More than 80 percent of these veterans can be linked to the 1900 census where information on their socioeconomic conditions circa 1900 can be found. Figure 2 delineates the life course of the veterans where information on year, mean age, and number of veterans alive at different time points has been presented. As indicated, early-life conditions (the period between birth and enlistment) have been related to chance of survival in the post-1900 periods. 3,976 veterans died between 1900 and 1910, and another 4,423 died between 1910 and 1920. The final two veterans in the sample died in 1946.
Figure 2.
The Life Course of Union Army Veterans in the Sample
Source: The Union Army Sample.
Analysis of possible sample selection bias indicates that the Union Army sample is generally representative of the population of white recruits into the Union Army. During the Civil War, approximately 95 percent of white males between age 18 and 25 in the United States were examined and approximately 75 percent of the examinees were inducted (Fogel, Engerman, and Floud 1983). Comparisons between the Union Army sample and the northern population in the same age group suggest these two groups resemble each other in terms of wealth in 1850 and 1860 and in terms of mortality circa 1900 (Fogel et al. 2001). The wealthiest whites in the early 1860s, however, might be less represented in the sample since some of them could have paid a substitution fee to have others enlist for them.2
An important issue concerns the handling of missing values for the variables used in this study. For most of the variables used, the proportion of cases with missing values is less than 10 percent. Due to the difficulties encountered in linking the Union Army records with the 1900 census, however, socioeconomic variables such as occupation in 1900, marital status, owning or renting house and so forth have a significant proportion of cases with missing values, ranging from 18 to 41 percent. In this study, to retain most cases for data analysis, missing values for these variables have been coded as “unknown” so that these cases can still stay in the analysis, as indicated in Table 1 where variables characterizing risk factors early in life and SES circa 1900 have been listed.
Table 1.
Description of the Variables Used in the Analysis (N=11,978)
| Risk Factors in Early Life | Mean or % | SES Circs 1900 | Mean or % |
|---|---|---|---|
| Birth Year | 1839.0 | Age in 1900 | 61.2 |
| Birth Season | Occupation in 1900 | ||
| Spring (March to May) | 26.9 | Farmer | 24.7 |
| Summer (June to August) | 23.7 | Artisan | 8.5 |
| Autumn (Sept. to Nov.) | 24.1 | Manual Labor | 7.5 |
| Winter (Dec. to Feb.) | 25.3 | Professional | 10.8 |
| Birth Country | Other | 7.9 | |
| United States | 83.2 | Unknown | 40.5 |
| Britain | 2.7 | Marital Status in 1900 | |
| Canada | 2.7 | Married | 73.5 |
| Germany | 5.7 | Single | 4.1 |
| Ireland | 3.7 | Widowered | 9.6 |
| Other Countries | 2.0 | Divorced | 0.7 |
| Coming from Big Cities1 | 6.6 | Unknown | 12.1 |
| Residential Regions2 | Literacy in 1900 | ||
| North Atlantic | 27.5 | Able to Read | 77.3 |
| South Atlantic | 4.3 | Unable to Read | 4.1 |
| North Central | 58.3 | Unknown | 18.6 |
| South Central | 4.1 | Own or Rent House in 1900 | |
| Other | 5.8 | Own | 50.9 |
| Wealth in 18603 | Rent | 20.0 | |
| Personal Property Value ($) | 395.2 | Unknown | 29.2 |
| Real Estate Value ($) | 1171.7 | Age at Death | 75.9 |
| Height at Enlistment (Inch) | 67.6 |
Source: The Union Army Sample.
Notes:
Defined as one of the 25 largest cities in 1860 with a minimum population of 37,000 based on data from the 1860 Census.
North Atlantic: Connecticut, Massachusetts, Maine, New Hampshire, New Jersey, Pennsylvania, Rhode Island, and Vermont.
South Atlantic: District of Columbia, Delaware, Florida, Georgia, Maryland, North Carolina, and South Carolina.
North Central: Iowa, Illinois, Indiana, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin.
South Central: Alabama, Arkansas, Kentucky, Louisiana, and Mississippi.
Other states include most western states along with Alaska and Hawaii.
Wealth in 1860 is by U.S. dollar in 1860 value and is only available for those veterans who were already married in 1860 and who have been successfully linked to the 1860 census.
The Union Army sample provides rich information on veterans’ life experiences prior to enlistment. About 80 percent of the veterans were born between 1830 and 1845. Based on information on veterans’ birth dates, season of birth has been coded as: March to May for spring, June to August for summer, September to November for autumn, and December to February for winter. In terms of country of birth, 83 percent of the veterans were native-born, 5.7 percent were born in Germany, and 3.7 percent in Ireland. About seven percent of the veterans came from one of the 25 largest cities in 1860, with a minimum population of 37,000. More than 85 percent of the veterans lived in the North Central and North Atlantic regions prior to enlistment. Based on information from the 1860 census, the average value of personal property for a married veteran is about $400; the average value of real estate is about $1,172. Height at enlistment averages at 67.6 inches.
The Union Army sample also incorporates variables on veterans’ SES circa 1900 when the average age for survived veterans was 61 with 80 percent of them between age 55 and 70. For about 41 percent of the veterans, information on their occupation circa 1900 is missing. Since more than half of the veterans in 1900 were at age 60 or above, a substantial proportion of them could have been out of labor force by then, which can partially explain why so many veterans failed to report their occupation in the 1900 census.3 About 74 percent of the veterans were married in 1900, four percent single, 10 percent widower, less than one percent divorced, and 12 percent with missing information. Seventy seven percent of the veterans reported they could read and four percent said they couldn’t, with the rest unknown. Twenty percent of the veterans rented houses or apartments, 51 percent owned their houses, and the rest unknown.
Methods
The dependent variable is the hazard rate of dying at any time after 1900 given that a veteran has survived to 1900, which is modeled in the Cox Proportional Hazard (CPH) analysis as a function of age in 1900 and exposures to risk factors prior to enlistment as well as circa 1900. Mathematically, the CPH modeling can be expressed as:
Where:
h(t) = the hazard function at time t. In this study it represents mortality risk at time t.
h0(t) = the baseline hazard, resembling the constant in multivariate regression analysis
x1, x2… xn = explanatory variables in the model
b1, b2…bn= estimated coefficients in the CPH model
An important assumption of the CPH analysis is the proportionality of hazard, that is, the effect of changing values for a certain explanatory variable on the hazard rate is constant, independent of time. This assumption was tested for all the explanatory variables in the CPH models using the ‘sthphest’ command in STATA, a test that relates the Schoenfeld residuals to time. The test results indicate that the proportionality assumption holds for the explanatory variables that have been used to characterize veterans’ risk exposures in early life.
Survival after 1900 was decomposed into three periods: between 1900 and 1910, between 1910 and 1920, and after 1920. The decomposition allows for an examination of the extent to which the effects of risk exposures in veterans’ early life on their chance of survival in old age changes as they become progressively older. Moreover, for each of the three periods after 1900 two CPH models were used to relate risk exposures in early life to chance of survival in old age, with one incorporating only risk factors prior to enlistment as the explanatory variables and the other including risk factors both early in life and circa 1900. Comparisons between the two CPH models are expected to reveal whether and to what extent an incorporation of SES in later life modifies the effects of early-life risk exposures on chance of survival in old age. These specifications gave rise to six CPH models constituting a sensitivity analysis of the effects of risk exposures in early life on old-age survival.
RESULTS
Table 2 shows the analytical results from the six CPH models where the hazard ratios as well as their 95 percent confidence intervals are presented. Whereas Models 1, 3, and 5 only use risk factors in early life to predict chance of survival in the three periods after 1900, coefficients from Models 2, 4, and 6 reveal the net effects of risk factors in early life on old-age survival in the corresponding periods after adjusting for the effects of SES circa 1900 as characterized by occupation, marital status, literacy, and homeownership.
Table 2.
Risk Exposures in Early Life and post-1900 Mortality Risk among Union Army Veterans
| Explanatory Variables | Hazard Ratios | |||||
|---|---|---|---|---|---|---|
| Between 1900 and 1910 |
Between 1910 and 1920 |
After 1920 |
||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Age in 1900 | 1.09*** (1.08, 1.09) | 1.09*** (1.08, 1.09) | 1.09*** (1.08, 1.09) | 1.09*** (1.08, 1.10) | 1.07*** (1.06, 1.08) | 1.07*** (1.06, 1.08) |
| Birth Season | ||||||
| Spring | Reference | Reference | Reference | Reference | Reference | Reference |
| Summer | 0.96 (0.87, 1.06) | 0.97 (0.88, 1.06) | 1.02 (0.94, 1.12) | 1.03 (0.94, 1.12) | 1.03 (0.93, 1.14) | 1.02 (0.92, 1.13) |
| Autumn | 0.96 (0.87,1.06) | 0.96 (0.87,1.05) | 0.91* (0.84,1.00) | 0.92* (0.84,1.00) | 0.96 (0.87,1.06) | 0.96 (0.87,1.06) |
| Winter | 0.92 (0.83,1.01) | 0.91 (0.83,1.01) | 1.00 (0.92,1.09) | 1.00 (0.92,1.09) | 1.01 (0.92,1.12) | 1.01 (0.91,1.12) |
| Birth Country | ||||||
| United States | Reference | Reference | Reference | Reference | Reference | Reference |
| Britain | 1.07 (0.87,1.31) | 1.07 (0.88,1.31) | 1.08 (0.89,1.30) | 1.09 (0.90,1.32) | 0.86 (0.67,1.10) | 0.86 (0.67,1.10) |
| Canada | 1.00 (0.80,1.24) | 1.01 (0.81,1.26) | 1.03 (0.85,1.24) | 1.02 (0.84,1.23) | 1.01 (0.80,1.25) | 1.00 (0.80,1.25) |
| Germany | 1.06 (0.92,1.21) | 1.08 (0.94,1.24) | 1.14* (1.00,1.31) | 1.15* (1.01,1.32) | 1.08 (0.89,1.31) | 1.08 (0.89,1.31) |
| Ireland | 1.39*** (1.18,1.62) | 1.40*** (1.19,1.64) | 1.01 (0.84,1.22) | 0.99 (0.82,1.19) | 1.27* (1.00,1.61) | 1.24 (0.97,1.57) |
| Other Countries | 1.01 (0.80,1.28) | 1.02 (0.81,1.29) | 0.83 (0.65,1.05) | 0.83 (0.65,1.06) | 0.76* (0.59,0.99) | 0.76* (0.58,0.99) |
| Coming from Big Cities | 1.32*** (1.16,1.51) | 1.28*** (1.12,1.47) | 1.10 (0.96,1.26) | 1.08 (0.94,1.23) | 1.13 (0.96,1.32) | 1.13 (0.96,1.33) |
| Residential Regions | ||||||
| North Atlantic | Reference | Reference | Reference | Reference | Reference | Reference |
| South Atlantic | 0.93 (0.78,1.11) | 0.93 (0.77,1.12) | 0.81* (0.69,0.95) | 0.83* (0.70,0.98) | 0.96 (0.79,1.16) | 0.95 (0.78,1.15) |
| North Central | 0.90** (0.83,0.98) | 0.91* (0.84,0.98) | 0.85*** (0.79,0.91) | 0.86*** (0.80,0.92) | 0.93 (0.85,1.01) | 0.93 (0.85,1.01) |
| South Central | 0.87 (0.72,1.05) | 0.88 (0.72,1.07) | 0.80** (0.68,0.94) | 0.82* (0.69,0.97) | 0.94 (0.78,1.13) | 0.93 (0.77,1.13) |
| Other | 0.67*** (0.56,0.80) | 0.71*** (0.59,0.85) | 0.75*** (0.65,0.86) | 0.76*** (0.66,0.87) | 0.85* (0.73,0.99) | 0.85* (0.73,0.99) |
| Height at Enlistment in Inches | 1.00 (0.98,1.01) | 1.00 (0.98,1.01) | 1.02* (1.00,1.03) | 1.02** (1.00,1.03) | 1.02** (1.00,1.04) | 1.02** (1.01,1.04) |
| Number of Cases | 10,430 | 10,430 | 7,131 | 7,131 | 3,025 | 3,025 |
Source: The Union Army Sample.
Notes:
p<0.05;
p<0.01;
p<0.001.
Coefficients from Models 2,4, and 6 show the effects of risk exposures in early life on old-age mortality after adjusting for the effects of socioeconomic factors circa 1900 including literacy, home ownership, occupation, and marital status.
Being born in autumn is associated with a significant survival advantage during the period between 1910 and 1920, but not for the other two periods. Results from Model 3 suggest that relative to those born in spring risk of mortality for veterans who were born in autumn is on average nine percent lower for the period between 1910 and 1920. This finding is consistent with previous studies where it has been found that being born in autumn or the fourth quarter is associated with lower risk of mortality (e.g. Doblhammer and Vaupel 2001; Lerchl 2004; Costa and Lahey 2005). The corresponding coefficient from Model 4 further indicates that the survival advantage for autumn-born veterans is robust irrespective of whether or not their SES circa 1900 has been taken into account.
Country of birth makes a significant difference in chance of survival in the post-1900 periods. This is especially the case for veterans who were born in Ireland. Relative to their U.S.-born counterparts, risk of mortality for Ireland-born veterans in the period between 1900 and 1910 is 39 percent higher. This effect persists after adjusting for the effects of SES circa 1900, suggesting the survival disadvantage associated with being born in Ireland has not been modified by SES in old age. A comparison between coefficients across the six CPH models further reveals that the Ireland-born survival disadvantage is most salient during the first decade after 1900, then it dissipates during the second decade, and finally it regains some of its salience after 1920.
A similar survival disadvantage has also been observed for Germany-born veterans. However, different from veterans born in Ireland, the survival disadvantage for Germany-born veterans becomes significant only for the period between 1910 and 1920. Results from Models 3 and 4 indicate that the elevated risk of mortality for Germany-born veterans in relation to native-born veterans is about 14 percent, regardless of whether or not the effects of SES circa 1900 have been taken into account. Given that Germany and Ireland were the two largest sources of foreign-born Union Army veterans, the findings here suggest that foreign-born veterans were in general not as healthy as their U.S.-born counterparts, which could be related to the economic distress they had in their native countries prior to their entry into the U.S.
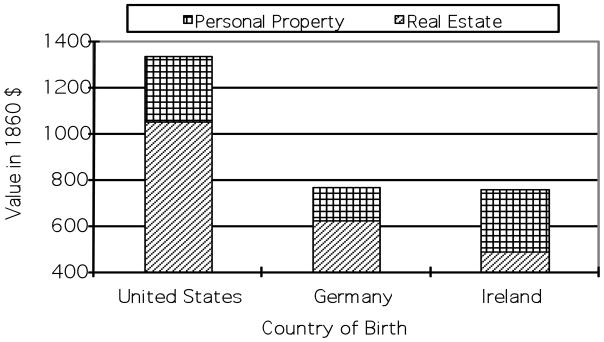
Even after their arrival in the United States, veterans from Ireland and Germany did not perform as well as native-born veterans in terms of economic well-being. Based on information from the 1860 census, Figure 3 shows the difference in wealth between veterans from these two countries and those born in the United States. Information on wealth is only available for some older veterans, most of whom had already been married by 1860 and who have been successfully linked to the 1860 census. The main economic advantage of US-born veterans lies in their ownership of real estate, the value of which is substantially higher than that for veterans from Germany and Ireland.
Figure 3.
Wealth in 1860 by Country of Birth
Source: The Union Army Sample.
Coming from big cities prior to enlistment is associated with a much higher risk of mortality in the first decade after 1900. Relative to those who did not come from big cities prior to enlistment, the elevated risk of mortality for veterans who were from big cities is 32 percent in Model 1 and 28 percent in Model 2. These two effects are highly significant and come close in size, suggesting that SES circa 1900 essentially did not modify the negative effects of coming from big cities on survival in old age.
The results from the CPH models also reveal a geographic pattern on risk of mortality among the veterans, with coming from North Atlantic region associated with a higher mortality risk as compared to coming from other regions prior to enlistment. Compared to veterans from North Atlantic region, mortality risk is reduced for veterans from South Atlantic, North Central, South Central, and Other region. This effect is particularly obvious in Models 3 and 4 when mortality risk from 1910 to 1920 is concerned, but it becomes attenuated in Models 5 and 6 when it comes to mortality risk in the post-1920 period.
Height at enlistment shows a positive correlation with risk of mortality after 1910. After adjusting for other explanatory variables in the CPH models, for each inch of increase in height, the relative risk of mortality increases by two percent. This significant effect of height persists across all four models for the two periods after 1910, suggesting that the height effect on veterans’ survival in old age has not been modified by SES circa 1900.
DISCUSSION
A discussion of the findings from this study and their implications cannot be possibly separated from the historical context of the nineteenth–century United States. About 85 percent of the Union Army veterans in the sample were born between 1825 and 1845, exposed to a far more adverse epidemiological environment all through their life compared to today’s Americans. The average number of chronic conditions per Union Army veteran at age 50 to 54 is three, as compared to one for American males in the same age group in the 1990s (Fogel 2004, p.91). In Massachusetts, death rates for respiratory tuberculosis declined from 365 per 100,000 in 1861 all the way to 37 per 100,000 in 1945, a remarkable reduction of 90 percent (Bureau of the Census 1949, p.48). Given the unprecedented improvement in the epidemiological environment since the nineteenth century, it becomes important to examine how exposures in early life were related to old-age mortality in the past and to what extent this relation has changed over time.
This study examines the relation between veterans’ risk exposures prior to enlistment and their chance of survival after 1900 under alternative model specifications. The results indicate that risk exposures in veterans’ early life–as approximated by birth season and country, city size, residential region prior to enlistment, and height at enlistment–significantly influence their chance of survival in old age. These effects are in general robust irrespective of whether or not SES in old age has been taken into account, implying that the main effects of risk exposures in early life on old-age survival cannot have been mediated through old-age SES.
Using longitudinal data from more recent cohorts, several studies have documented the relative importance of childhood living conditions and adulthood SES in health or mortality disparity in later life (e.g. Ben-Shlomo and Davey-Smith 1991; Marmot et al. 2001; Hayward and Gorman 2004; Luo and Waite 2005). A converging finding from these studies is that whereas exposures in early life are important in explaining health or mortality differentials in later life, adjusting for adulthood or current SES substantially mitigates or even abolishes the effects of risk exposures in earlier life. Such a finding contrasts with the results from this study where it has been revealed that the negative effects of risk exposures prior to enlistment on old-age mortality are robust regardless of whether or not SES in old age has been taken into account. The rigidity of the impacts of risk exposures earlier in life on adult mortality has also been documented in several other studies using historical samples (e.g. Bengtsson and Lindström 2003; Costa and Lahey 2005).
In light of these findings, the pooled evidence tends to suggest that risk exposures earlier in life play a more important role in mortality disparity at older ages among Union Army veterans than among more recent cohorts. In other words, the importance of early-life exposures in mortality disparity in late life seems to have declined over time. This is consistent with the fading importance of month of birth in mortality disparity over time (Doblhammer and Vaupel 2001). The rigidity of the impacts of risk exposures prior to enlistment on old-age survival among the veterans has to do with the harshness of living conditions in the nineteenth-century United States when malnutrition and infectious diseases posed grave threat to health and when massive immunization and effective treatments of diseases were unavailable. Veterans who were exposed to environmental insults during infancy or childhood could have a lifelong disadvantage in health, which cannot be easily modified or reversed by living conditions in adulthood. By contrast, for those from more recent cohorts whose living conditions in early life were substantially improved, the reduced level of risk exposures in early life opens a higher possibility for modifications or even reversals by living conditions in adulthood.
Findings from this study also suggest that most of the effects of risk exposures in early life on old-age survival are contingent on the specific age period in old age that has been selected for analysis. A decomposition of survival after 1900 into three periods, as this study has shown, provides insights into mortality disparities among the veterans. Whereas some of the effects became fully unfolded in the initial 10 years after 1900, others did not express themselves until after 1910. This implies that the seemingly inconsistent findings on the relation between early-life exposures and old-age survival in the current literature might not result from the relation per se, but from the different age periods in each sample. Comparisons across studies will become more meaningful if this age factor can be taken into account.
The survival advantage associated with being born in autumn, as revealed in this study, complements relevant findings from previous studies in several aspects (e.g. Doblhammer and Vaupel 2001; Bengtsson and Lindström 2003; Lerchl 2004; Costa and Lahey 2005). Similar findings were also reported, for example, in Doblhammer and Vaupel’s study of the effect of month of birth on mortality after age 50 utilizing a more recent sample of over one million cases from several countries. With a much smaller sample, this study still reveals the significance of the autumn-born effect as well as its magnitude, suggesting that the beneficial effect of being born in autumn might well have been more salient in historical populations than in more recent cohorts. More importantly, results from this study further indicate that the autumn-born effect on survival turns out to be more pronounced in certain age periods than in other, and the effect generally persists with or without adjusting for the effect of SES in later life.
The observation that being taller is related to a higher relative risk of mortality for veterans who managed to survive past 1910 stands in contrast with some previous findings that have suggested a negative correlation between height and mortality (e.g. Waaler 1984; Costa 1993; Jousilahti et al. 2000). For instance, Waaler (1984) examined the height-mortality association among a national sample of Norwegians between 1963 and 1975. His findings indicate that the mortality advantage associated with being taller declines with age. At age 70 and above, height virtually makes no difference in mortality among males. Waaler’s finding is consistent with other studies on the age patterning of mortality disparity where it has been indicated that socioeconomic disparity in adult mortality tends to be the largest during early adulthood or middle age, but dwindles into old age (House, Kessler, and Herzog 1990; Preston and Taubman 1994; Williams and Collins 1995; Beckett 2000). The main explanation of the decline in health disparity at old age is mortality selection; that is, socially disadvantaged people disproportionally die out before they could survive to old age.
An alternative explanation for the elevated mortality risk for taller veterans could lie in the possible survival advantages associated with being short per se. In a comprehensive review of the relation between height and longevity, Samaras et al. (2003) reported that shorter, smaller bodies have lower death rates and fewer diet-related chronic diseases, especially past middle age. This review also noted that smaller animals within the same species generally live longer. At the end of the review, Samaras et al. also put forward a counter-argument for the well-documented association between shorter stature and higher risk of cardiovascular diseases by pointing out that the association is caused more by Western lifestyle and diet than by short stature per se, since many populations of short people in Asia and Africa are almost free of cardiovascular diseases until Western lifestyle and dietary changes occur. When these changes do occur, the narrower coronary blood vessels of shorter people are more prone to clogging, which can eventually lead to an elevated risk of developing cardiovascular diseases.
The much elevated risk of mortality for veterans who were born in Ireland, and to a less extent for veterans born in Germany, is indicative of a significant health advantage of native-born veterans over their immigrant counterparts. This is consistent with the estimate that the American height advantage over Western and Northern Europeans was in between 3 to 9 centimeters in the middle of the nineteenth century (Komlos and Baur 2004). Similar health disparities between the native-born and immigrants from Ireland and Germany were also documented in Dublin and Baker’s report on mortality rates among different immigrant groups in the U.S. in the early twentieth century (Dublin and Baker 1920). Their findings suggest that immigrants from Ireland, Germany, and Britain, when compared to their fellow countrymen at home in the same period, had a higher risk of developing pneumonia, Bright’s disease, tuberculosis, and other diseases after they came to the U.S. This made Dublin and Baker question at the end of their paper whether the immigrants from these three countries represent the best of their native populations.
“The much more favorable economic conditions under which they live in the United States than in their own countries should result in lower death rates. But in several instances we found that this does not prevail; the facts indicate, on the whole, deterioration rather than improvement. Is it possible that our immigrants are not representative of the best in their native countries?” (p. 44)
Part of the answer to this question lies in the observation that the majority of immigrants from these three countries came to the U.S. in mid nineteenth century to flee economic distresses in their native countries where the poor were suffering the most. Based on information from the passenger records from 119 ships that arrived at New York City between 1836 and 1853, Cohn (1992) examined the occupational distribution among British immigrants. The findings suggest that laborers appear to be the dominant group of immigrants, which according to Cohn, supports the view that distress was the most important cause of immigration. If most of the immigrants from Britain, Ireland, and Germany were relatively poor compared to the rest of their native population, they could also be worse off in terms of health status as Dublin and Baker have found. This selectivity of immigration also implies that for many young Irish, German, and British immigrants who came to the U.S. circa the mid nineteenth century, their disproportional exposures to economic hardship and other environmental insults during infancy or childhood before they came to the U.S. can be a long lasting liability for them and negatively influence their chance of survival in old age. This trend, however, got reversed in the late twentieth century when both male and female immigrants had longer life expectancy than US-born Americans (Singh and Miller 2004).
LIMITATIONS AND DIRECTIOS FOR FUTURE RESEARCH
One of the core findings of this study is that the effect of risk exposures early in life on chance of survival is sensitive to the particular age periods in later life that have been selected for analysis, an issue that has largely been neglected by previous studies. Despite the importance of the finding, this study touches little on the ‘why’ questions. For example, why should it be the case that the negative effect of being born in Ireland became fully unfolded in the period between 1900 and 1910, whereas a similar effect of being born in Germany did not peak until after 1910? Answering this question requires a closer look into relevant life events of the two immigrant groups that could potentially explain the observed timing differentials. For this purpose, it would be useful for future studies to disentangle the age, cohort, and period effects and assess their contribution to the observed differentials in timing.
The finding on the positive association between height and risk of mortality among the veterans in the post-1910 period deserves further inquiry. In a harsh epidemiological environment mortality selection could potentially reverse the well documented negative association between height and mortality. This selection explanation can only be verified by examining mortality rates by height among the veterans prior 1900, for which the current Union Army sample might have a sample selection bias. The U.S. Congress originally established the basic system of pension laws, called the General Law, and limited the benefit to those who could prove their disabilities were a result of the war. Later, in 1890, the Disability Act was approved, marking the beginning of a more universal pension program that only required pensioners to have served in the military for 90 days. This implies that holding other factors constant, veterans who died before 1890 on average had a lower chance of being selected into the Union Army sample than those who died after 1890.
An important limitation of this study is that no information on diseases and cause-specific mortality has been incorporated into the analyses. To a large extent the diseases that veterans developed in late life can be regarded as the proximate causes of their deaths. The exclusion of socioeconomic well-being circa 1900 as the pathway through which early risk exposures influence chance of survival in old age, as suggested by this study, further points to the possibility that more of the effects of earlier exposures on old-age mortality could be mediated through their effects on the development of chronic conditions in old age. Future studies can assess how veterans’ risk exposures prior to enlistment and during wartime influence their risk of developing chronic diseases, becoming disabled, or dying from a certain disease or a combination of diseases in old age. A complicated issue confronting these studies concerns the coding of diseases and causes of death, as well as dealing with cases with missing information on these variables in the Union Army sample. Given the importance of these information and the additional insights they provide, however, such an endeavor would be well worth it.
There is no doubt that more information is needed to better characterize veterans’ epidemiological environment prior to enlistment. Season of birth, country of origin, city size, residential regions, and height as used in this study provide some information about veterans’ initial risk exposures, but they are far from enough. Previous studies have also documented the role of epidemiological environment during infancy, as approximated by infant mortality rates, in mortality differentials in later life. For example, Bengtsson and Lindström (2003) assessed the importance of life conditions circa birth and socioeconomic conditions during adulthood to mortality at ages 55 to 80, using historical data collected from four rural parishes in southern Sweden from 1766 to 1894. They found that after adjusting for socioeconomic status in later life, the disease load experienced during the birth year, as indicated by infant mortality rates, was significantly associated with old-age mortality. Specifically, exposure to airborne infectious diseases during the first year of life substantially increased mortality at ages 55 to 80. If further information on infant mortality rates, famines, infectious diseases, source of drinking water, terrain features and so forth at the city or county level becomes available, veterans’ exposures to risk factors in early life can be more accurately characterized. Besides, the use of residential regions and city size as fixed covariates can be inadequate to capture risk exposures for veterans who moved between cities or between rural and urban areas.
Finally, while country of origin provides a proxy for veterans’ exposure to economic distress prior their migration to the United States, there is no record on when and at what ages the veterans migrated to the U.S. The timing of immigration is important because it allows for a consideration of the effect of when and for how long the veterans experienced economic distress in their countries of origin. It seems reasonable to presume that the impact of risk exposure would be more pronounced for veterans who experienced the hardship during critical periods (for example, infancy) of development than for those who had similar hardship but during a non-critical period of growth.
Acknowledgments
This study is supported by a grant from the National Institute of Aging, AG10120-09A1. I thank Robert Fogel, Linda Waite, Patrick Heuveline, and Tom Chappelear for their comments.
Footnotes
A previous version of the paper was presented at the 2007 Annual Meeting of the Population Association of America, New York.
This percentage was calculated by the author based on relevant information from the 2002 mortality report by the National Center for Health Statistics of the United States. http://www.cdc.gov/nchs/fastats/pdf/mortality/nvsr53_05t03.pdf. Accessed June 2008.
Based on information from the 1860 census, Wilcox (1992) presented the antebellum wealth distribution across occupational groups. Comparison between his finding and what I have found in the Union Army Sample suggests that professionals and proprietors in the Union Army sample were not as wealthy as their counterparts in the general population.
Based on the Wilcox codes derived from studies of labor force distribution in the antebellum economy (Wilcox, 1992), the farmer category includes both farmers and farm labors. In terms of the nature of work, farm labors resemble manual labors since both require substantial use of muscle power. However, since farm labors work in rural areas and most manual labors work in urban settings–two distinctive epidemiological environments in the nineteenth century U.S.–I still classify farm labors to the farmer category. The artisan category includes a wide variety of skilled workers such as carpenters, metal workers, foundry workers, brewers, bakers, sausage makers, and so forth. Manual laborers are those who essentially rely on their physical strength and muscle power such as miners and sailors. Professionals refer to those who owned a business or with certification or substantial expertise in their career, such as lawyers, doctors, professors and teachers, landlords, dealers, and so forth. Finally, all the rest have been classified into ‘other’ category, which includes servants, the unemployed, and those with occupations unclassifiable or unidentifiable.
Given the large size of the ‘unknown’ category in occupational distribution in 1900, I compared veterans with occupation known to those with occupation unknown in 1900. The comparison suggests that these two groups of veterans resemble each other in terms of age in 1900, age at death, height at enlistment, percentage wounded during war, and occupational distribution at enlistment, although veterans with occupation unknown in 1900 have a higher proportion of being born in a foreign country.
References
- Avchen RN, Scott KG, Mason CA. Birth weight and school-age disabilities: a population-based study. American Journal of Epidemiology. 2001;154(10):895–901. doi: 10.1093/aje/154.10.895. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Health in Later Life. 2. Edinburgh, London, New York: Churchill Livingstone; 1998. [Google Scholar]
- Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. In: Barker DJP, editor. Fetal and Infant Origins of Adult Disease. London: British Medical Journal; 1992. pp. 23–27. [Google Scholar]
- Beckett M. Converging health inequalities in later life-an artifact of mortality selection? Journal of Health and Social Behavior. 2000;41:106–119. [PubMed] [Google Scholar]
- Ben-Shlomo Y, Davey-Smith G. Deprivation in infancy or in adult life: which is more important for mortality risk? Lancet. 1991;337:530–4. doi: 10.1016/0140-6736(91)91307-g. [DOI] [PubMed] [Google Scholar]
- Bengtsson T, Lindström M. Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766–1894. International Journal of Epidemiology. 2003;32(2):286–294. doi: 10.1093/ije/dyg061. [DOI] [PubMed] [Google Scholar]
- Blackwell DL, Hayward MD, Crimmins EM. Does childhood health affect chronic morbidity in later life? Social Science and Medicine. 2001;52:1269–1284. doi: 10.1016/s0277-9536(00)00230-6. [DOI] [PubMed] [Google Scholar]
- Bureau of the Census. Historical Statistics of the United States, 1789–1945: A Supplement to the Statistical Abstract of the United States. Washington, D.C: 1949. [Google Scholar]
- Cohn Raymond L. Mortality on immigrant voyages to New York, 1836–1853. Journal of Economic History. 1984;44:289–300. doi: 10.1017/s0022050700031892. [DOI] [PubMed] [Google Scholar]
- Cohn Raymond L. The occupations of English immigrants to the United States, 1836–1853. The Journal of Economic History. 1992;52:377–387. [Google Scholar]
- Costa DL. Height, weight, wartime distress, and older age mortality: evidence from the Union Army Records. Explorations in Economic History. 1993;30:424–449. [Google Scholar]
- Costa D, Lahey J. Becoming oldest-old: evidence from historical U.S. data. Genus. 2005;LXI(1):125–161. [Google Scholar]
- Davey-Smith G, Gunnell D, Ben-Shlomo Y. Life-course approaches to socioeconomic differentials in cause-specific adult mortality. In: Leon D, Walt G, editors. Poverty Inequality and Health: an International Perspective. New York: Oxford University Press; 2001. pp. 88–124. [Google Scholar]
- Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proceedings of the National Academy of Sciences. 2001;98(5):2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublin LI, Baker GW. The Mortality of Race Stocks in Pennsylvania and New York, 1910. Quarterly Publications of the American Statistical Association. 1920;17(129):13–44. [Google Scholar]
- Elo Irma T, Preston SH. Effects of early-life conditions on adult mortality: a review. Population Index. 1992;58:186–212. [PubMed] [Google Scholar]
- Fogel RW. New sources and new techniques for the study of secular trends in nutritional status, health, mortality, and the process of aging. Historical Methods. 1993;26:5–43. [Google Scholar]
- Fogel RW. Public Use Tape on the Aging Veterans of the Union Army: Federal Census Records, 1850, 1860, 1900, 1910 Version C-3. Center for Population Economics University of Chicago, Graduate School of Business, and Department of Economics. Brigham Young University; 2000. [Google Scholar]
- Fogel RW. Public Use Tape on the Aging Veterans of the Union Army: Military Pension and Medical Records, 1860–1940 Version M-5. Center for Population Economics University of Chicago, Graduate School of Business, and Department of Economics. Brigham Young University; 2000. [Google Scholar]
- Fogel RW. Public Use Tape on the Aging Veterans of the Union Army: Surgeon’s Certificates, 1862–1940. Version S-1 Standardized. Center for Population Economics University of Chicago, Graduate School of Business, and Department of Economics. Brigham Young University; 2001. [Google Scholar]
- Fogel RW. The Escape from Hunger and Premature Death 1700–2100. New York: Cambridge University Press; 2004. [Google Scholar]
- Fogel RW, Engerman SL, Floud R. Secular changes in American and British stature and nutrition. The Journal of Interdisciplinary History. 1983;14(2):445–81. [PubMed] [Google Scholar]
- Fogel RW, Costa DL. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography. 1997;34(1):49–66. [PubMed] [Google Scholar]
- Fogel RW, et al. Grant submitted to National Institutes of Health. Center for Population Economics, Graduate School of Business, University of Chicago; 2001. Early Indicators of Later Work Levels, Disease and Death. Typescript. [Google Scholar]
- Haines MR. The urban mortality transition in the United States, 1800–1940. Annales de Demographie Historique. 2001;2001:33–64. [Google Scholar]
- Hayward MD, Gorman BK. The long arm of childhood: the influence of early-life social conditions on men’s mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. Milbank Quarterly. 1990;68(3):383–411. [PubMed] [Google Scholar]
- Jousilahti P, Tuomilehto J, Vartiainen E, Eriksson J, Puska P. Relation of adult height to cause-specific and total mortality: a prospective follow-up study of 31,199 middle-aged men and women in Finland. American Journal of Epidemiology. 2000;151(11):1112–20. doi: 10.1093/oxfordjournals.aje.a010155. [DOI] [PubMed] [Google Scholar]
- Komlos J, Baur M. From the tallest to (one of) the fattest: the enigmatic fate of the American population in the 20th Century. Economics and Human Biology. 2004;2(1):57–74. doi: 10.1016/j.ehb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Koelle K, Pascual M, Yunus M. Pathogen adaptation to seasonal forcing and climate change. Proceedings of the Royal Society, Series B, Biological Sciences. 2005;272:971–977. doi: 10.1098/rspb.2004.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Prior exposure to disease and later health and mortality: evidence from Civil War records. In: Costa DL, editor. Health and Labor Force Participation over the Life Cycle: Evidence from the Past. Chicago: University of Chicago Press; 2003. pp. 51–88. [Google Scholar]
- Lerchl A. Month of birth and life expectancy: role of gender and age in a comparative approach. Naturwissenschaften. 2004;91(9):422–5. doi: 10.1007/s00114-004-0553-5. [DOI] [PubMed] [Google Scholar]
- Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. Journals of Gerontology-Series B: Psychological Sciences and Social Sciences. 2005;60(2):S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Shipley M, Brunner E, Hemingway H. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II Study. Journal of Epidemiology and Community Health. 2001;55(5):301–7. doi: 10.1136/jech.55.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn CN, Barker DJP, Osmond C. Mothers’ pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. The Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- Myers GC. Mortality and health dynamics at older ages. In: Ruzicka L, Wunsch G, Kane P, editors. Differential Mortality: Methodological Issues and Biosocial Factors. Oxford: Clarendon Press; 1989. [Google Scholar]
- Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. The Milbank Memorial Fund Quarterly. 1971;49(4):509–38. [PubMed] [Google Scholar]
- Preston SH, Haines MR. Fatal Years: Child Mortality in Late Nineteenth- Century America. Princeton: Princeton University Press; 1991. [Google Scholar]
- Preston SH, Taubman P. Socioeconomic differences in adult mortality and health status. In: Martin L, Preston SH, editors. The Demography of Aging. Washington, DC: National Research Council; 1994. [Google Scholar]
- Riley J. Height, nutrition and mortality risk reconsidered. Journal of Interdisciplinary History. 1994;24(3):465–492. [Google Scholar]
- Riley J. Rising Life Expectancy: A Global History. New York: Cambridge University Press; 2001. [Google Scholar]
- Samaras TT, Elrick H, Storms LH. Is height related to longevity? Life Sciences. 2003;72:1781–1802. doi: 10.1016/s0024-3205(02)02503-1. [DOI] [PubMed] [Google Scholar]
- Singh GK, Miller BA. Health, life expectancy, and mortality patterns among immigrant populations in the United States. Canadian Journal of Public Health. 2004;95(3):114–121. doi: 10.1007/BF03403660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HT, Sabroe S, Rothman KJ, Gillman M, Steffensen FH, Fischer P, Sorensen TIA. Birth weight and length as predictors for adult height. American Journal of Epidemiology. 1999;149(8):726–9. doi: 10.1093/oxfordjournals.aje.a009881. [DOI] [PubMed] [Google Scholar]
- Spira WM. Environmental factors in diarrhea transmission: the ecology of Vibrio cholerae 01 and cholera. In: Holme T, Holmgren J, Merson MH, Mollby R, editors. Acute Enteric Infections In Children: New Prospects for Treatment and Prevention. Amsterdam: Elsevier/North-Holland Biomedical Press; 1981. [Google Scholar]
- Troesken W. NBER Working Paper 9549. 2003. Lead water pipes and infant mortality in turn-of-the century Massachusetts. [Google Scholar]
- Waaler H. Height, weight and mortality. The Norwegian experience. Acta medica Scandinavica Supplementum. 1984;679:1–56. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- Watson PE, McDonald BW. Seasonal variation of nutrient intake in pregnancy: effects on infant measures and possible influence on diseases related to season of birth. European Journal of Clinical Nutrition. 2007;61(11):1271–80. doi: 10.1038/sj.ejcn.1602644. [DOI] [PubMed] [Google Scholar]
- Wilcox N. A note on the occupational distribution of the urban United States in 1860. In: Fogel RW, Galantine RA, Manning RL, editors. Without Consent or Contract: The Rise and Fall of American Slavery, vol. 2, Evidence and Methods. New York: W. W. Norton and Company; 1992. [Google Scholar]
- Williams DR, Collins C. U.S. socioeconomic and racial differences in health. Annual Review of Sociology. 1995;21:349–386. [Google Scholar]
- Wilson SE. The prevalence of chronic respiratory disease in the industrial era: the United States, 1895–1910. In: Costa DL, editor. Health and Labor Force Participation over the Life Cycle: Evidence from the Past. Chicago: The University of Chicago Press; 2003. pp. 147–180. [Google Scholar]
- World Health Organization. [Accessed June 2008.];Emerging Issues in Water an Infectious Disease. 2003 Available at ≪ http://www.who.int/water_sanitation_health/emerging/emerging.pdf.≫.