Fig. 6.
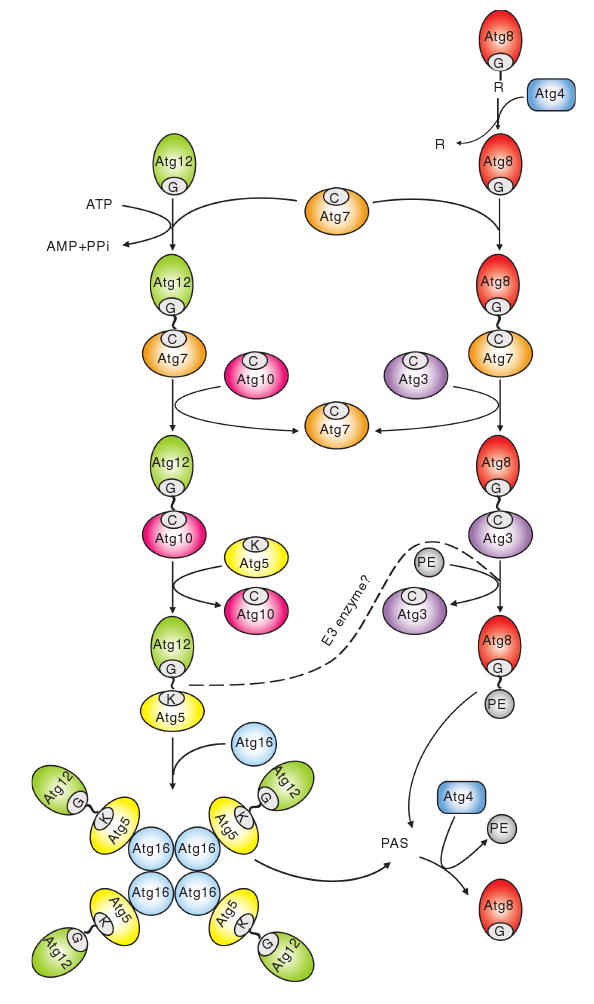
Two ubiquitin-like protein conjugation systems. The conjugation of Atg12 to Atg5 starts with activation by Atg7, which is homologous to the E1 ubiquitin-activating enzyme. Atg7 hydrolyzes ATP, resulting in the activation of Atg12 via the formation of a thioester bond between the C-terminal glycine of Atg12 and the active site cysteine of Atg7; subsequently, the activated Atg12 is transferred to the active site cysteine of Atg10, an E2-like enzyme, which catalyzes the conjugation of Atg12 to Atg5 through the formation of an isopeptide bond between the activated glycine of Atg12 and an internal lysine residue of Atg5. Atg12—Atg5 is finally assembled with Atg16. Atg16 forms a tetramer to allow the formation of an Atg12—Atg5—Atg16 multimeric structure. The conjugation of Atg8—PE starts with the cleavage of the C-terminal arginine of Atg8 by the protease Atg4. The exposed glycine of Atg8 is then bound to the active site cysteine of the same E1-like enzyme, Atg7. The activated Atg8 is then transferred to another E2-like enzyme, Atg3. Eventually, Atg3 catalyzes the conjugation of Atg8 to form Atg8—PE. The Atg12—Atg5 conjugate might function as an E3, ubiquitin ligase-like enzyme, to promote Atg8—PE conjugation. Both the Atg12—Atg5—Atg16 complex and Atg8—PE localize to the PAS to facilitate vesicle formation. The Atg8—PE that resides on the outer face of the sequestering vesicle is released from the membrane by a second Atg4-dependent cleavage. This figure is modified from Fig. 4 of Yorimitsu and Klionsky (2005b)
