Figure 3.
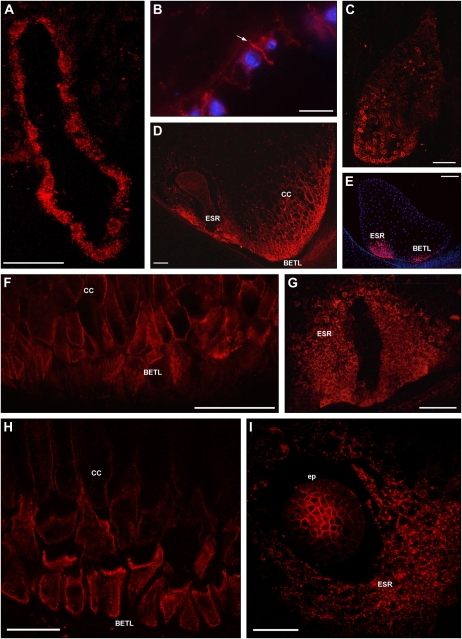
ZmPIN1 protein localization during maize endosperm development. All images portray maize B73 kernel sections labeled with the anti-AtPIN1 antibody plus a secondary antibody carrying the Alexa568 fluorochrome. A, H, and I present laser confocal images, while B to G present epifluorescence images acquired with a Leica DC300F camera. Nuclei in B and E are blue due to DAPI staining. During the first events of endosperm development, ZmPIN1 proteins are detectable in the nuclear cytoplasmic domains of syncytial endosperm, where proteins are localized in endomembrane systems (A). ZmPIN1 proteins mark the formation of the first anticlinal cell plasma membrane at the beginning of the cellularization phase, while endomembranes of the syncytium are still labeled (B). The arrow in B indicates the newly formed anticlinal plasma membrane. At the end of the cellularization phase, ZmPIN1 proteins are detectable both in endomembranes and cell plasma membranes without any detectable polarity (C). A gradient of protein expression is evident: the proteins are more abundant in the basal-chalazal region of the endosperm (C), where BETL and ESR will differentiate. Both transfer layer and ESR cells show high ZmPIN1 levels (D and E), but these domains are characterized by two different ZmPIN1 localization patterns. In BETL and inner conductive cells, the proteins are localized in all the cell membranes, without any detectable polarization, suggesting a homeostasis of auxin levels between the cells of this endosperm domain (D, F, and H). In ESR, ZmPIN1s are detectable almost exclusively in the cell endomembranes (D, G, and I), suggesting a lack of auxin efflux mediated by PIN1 proteins from these cells. CC, Conductive cells; ep, embryo proper. Bars = 200 μ m in E; 100 μ m in A, C, D, F, and G; 75 μ m in I; 50 μ m in B; and 40 μ m in H.