Figure 3.
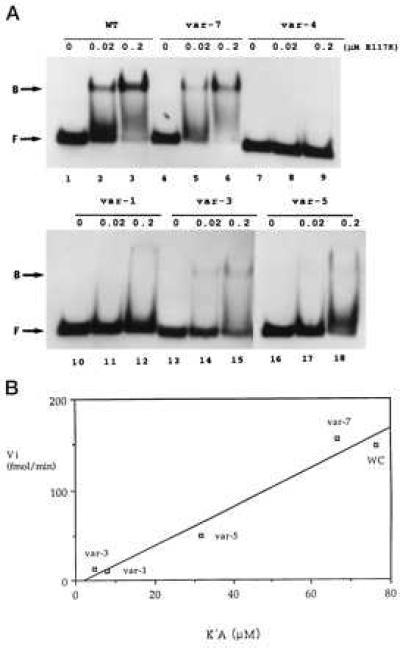
W-C bp substitution inhibits RNase III binding. (A) Gel shift assay of R1.1[WC] RNA variant binding to RNase III. 5′-32P-labeled RNA (104 dpm; 1.4 fmol) was combined with 0, 0.02, or 0.2 μM of the [E117K] RNase III mutant, then electrophoresed in a nondenaturing gel. (Upper) Lanes 1–3, R1.1[WC] RNA (WT); lanes 2–4, variant 7; and lanes 7–9, variant 4. (Lower) Lanes 1–3, variant 1; lanes 4–6, variant 3; and lanes 7–9, variant 5. (B) Correlation between initial cleavage rate (Vi) and binding affinity (1/K′D). WC refers to R1.1[WC] RNA. The Vi values are expressed as fmol product formed per minute (37°). The measured K′D values (nM ± SD) are the average of at least three experiments, and are: R1.1[WC] RNA, 13.1 ± 9.3; var-7, 15.0 ± 4.0; var-5, 31.5 ± 16.5; var-1, 126 ± 30; and var-3, 213 ± 117. Given the similarity in cleavage rates (Fig. 2A), the binding affinity of variant 2 was assumed to be similar to that for variant 3. The weak binding affinities of variants 4 and 6 prevented K′D determination.
