Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) disorder characterized by chronic abdominal discomfort, including pain, bloating and changes in bowel habits. The exact cause of IBS is not entirely understood. Recent studies have shown that IBS may be associated with altered serotonin (5-hydroxytryptamine, 5-HT) levels within the GI tract. About 90% of 5-HT in the human body is produced and stored in enterochromaffin (EC) cells that reside in the mucosal layer of the intestine. Measurements of serotonin availability locally in the mucosa can provide insight on the functionality of these cells and potentially the pathophysiology of the disease. In this study, we used continuous amperometry with a diamond microelectrode to record serotonin levels in vitro in the ileum mucosa as an oxidation current. The boron-doped diamond (BDD) microelectrode is quite practical for these measurements because if its low background signal, low sensitivity to solution pH changes, and excellent resistance to fouling by adsorbed serotonin oxidation reaction products. In fact, the measurements are only possible because of the unique properties of diamond. We present electrochemical data that demonstrate the diamond microelectrode’s utility for assessment of enterochromaffin cell function. Confirmation that the oxidation current was associated with indogenous serotonin release came from pharmacological studies. We are hopeful that these types of in vitro electrochemical measurements will lead to a better understanding of the pathophysiology of IBS.
1. Introduction
Boron-doped diamond (BDD) electrodes have been employed in electrochemical measurements for more than a decade now [1,2]. Type types of electrode architectures are typical: planar macroelectrodes and microelectrodes. Only recently, have diamond microelectrodes been shown to provide useful information about electroactive neurosignaling molecules in in vitro and in vivo measurements [3-6]. Diamond microelectrodes possess some superior properties compared to carbon fibers and metals. For example, the BDD electrodes exhibit low and stable background signals, a wide working potential window, excellent microstructural stability, good activity without conventional pretreatment, and resistance to fouling [7]. The last property is particularly desirable for in vivo or in vitro studies because many biomolecules strongly adsorb on the sp [2] carbon electrode surface, which makes monitoring of the release of electroactive molecules problematic [3]. In the recent years, studies have shown that BDD electrodes function well for real-time monitoring of 5-HT and norepinephrine in in vitro measurements [8,9].
5-HT is an important signaling molecule in the gastrointestinal tract [10]. Over 90% of the body’s 5-HT is synthesized and stored in the enterochromaffin (EC) cells located in the mucosa of the gut [11]. EC cells are sensory transducers that respond to mechanical or chemical stimuli (normally caused by a bolus of food) by releasing 5-HT. Most of the released 5-HT is cleared by the serotonin transporter (SERT) protein and transported into the SERT-expressing enterocytes [12]. Several selective serotonin reuptake inhibitors (SSRIs), such as citalopram or fluoxetine, can raise the extracellular 5-HT level in the gut by blocking SERT.
The EC cells and the enterocytes are localized together in the gut mucosa. Therefore, 5-HT signaling depends on the functionality of both the EC cells and SERT [11,12]. 5-HT acts at 5-HT receptors localized in mucosal endings of enteric and extrinsic primary afferent neurons to initiate motor reflexes and intestinal sensation [13]. Elevated levels of 5-HT in the GI tract have been associated with several gastrointestinal motility disorders [14,15], such as IBS [11], which affect 10 to 25% of the US population [16]. Local measurements of 5-HT availability can aid in the understanding of 5-HT handling in the enteric nerve system and ultimately may lead to new therapeutic approaches for treatment.
2. Experimental
2.1. Diamond microelectrode preparation
The boron-doped diamond thin film was deposited on a sharpened Pt wire using microwave-assisted chemical vapor deposition (CVD). The Pt wire (99.99%, Aldrich Chemicals, 76 μm diameter) was cut into a short piece 1.3 cm long. Both ends of the wire were electrochemically sharpened in 1 M KOH. The Pt wire was then ultrasonically cleaned in acetone for 10 minutes and ultrasonically seeded in a diamond powder suspension (3-6 nm particles, ca. 20 mg in 100 ml of ethanol, Tomei Diamond Co., Tokyo, Japan) for 30 minutes. The wire was suspended in the solution rather than allowed to rest on the bottom of the container to avoid damaging the sharpened tip. A thin film of boron-doped diamond was then deposited onto the wire using a commercial PECVD system (1.5 kW, 2.54 GHz, ASTeX, Woburn, MA, USA). The thin film was deposited from 0.5% CH4/H2 (v/v) source gas mixture with 10 ppm of diborane (0.1% B2H6 diluted in H2) added for doping. The microwave power was 600 W, the system pressure was 45 torr, and the substrate temperature was estimated to be between 600 to 800 °C using a single filament optical pyrometer. The electrode growth lasted 10 hours with multiple wires being coated during a run. Each of the diamond-coated Pt wires was then cut in the middle to produce 2 electrodes. Each electrode (the cut end) was affixed to a copper wire using conducting silver epoxy and insulated with polypropylene from a heated pipette tip [7]. The resulting microelectrode was a conically shaped with a tip about 10 μm in diameter and the cylinder about 80 μm wide. The exposed cone length was 300 to 400 μm. This insulation method sometimes resulted in electrodes partially covered with polypropylene, leading to a change in their electrochemical behaviors [17]. Electrodes were validated using cyclic voltammetry and several aqueous redox systems.
2.2. Voltammetry
Differential pulse voltammetry was used to measure 5-HT in standard solutions because the technique provides better sensitivity compared to other methods, such as cyclic voltammetry. A three electrode system, including a diamond microelectrode, a Pt wire counter electrode and a lab-made Ag|AgCl reference electrode, was employed. All measurements were carried out using a computer-controlled potentiostat (CH Instruments, model 650A, Austin, TX). In this experiment, the potential was scanned from -0.5 V to 1.2 V with a increase of 4 mV, amplitude of 50 mV, pulse width of 50 ms and sampling rate of 60 Hz. The diamond microelectrode was prepared for use by soaking in isopropyl alcohol (IPA) for several minutes and rinsed with deionized water thoroughly [18]. This pretreatment is effective at cleaning the diamond surface by dissolving and desorbing contaminants from the surface.
2.3. Tissue Preparation
All animal use protocols were approved by the Institutional Animal Use and Care Committee at Michigan State University. Adult male guinea pigs (3 weeks old, about 300 g) were obtained from Bioport, Inc. (Lansing, Michigan). A segment of the ileum was collected and place in an oxygenated (95% O2, 5% CO2) Krebs’ buffer solution (117 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol MgCl2, 1.2 mmol/L NaH2PO4, 25 mmol/L NaHCO3, 11 mmol/L glucose, pH=7.4). A small piece of ileum (about 1 cm long) was place in a flow chamber. The segment was cut open and pinned to the flow chamber with the mucosa side facing up. The flow chamber was then mounted on the stage of an inverted microscope (Olympus, CKX41, Tokyo, Japan). Krebs’ solution was constantly flowed through the chamber at 2 ml/min at room temperature. Tissues were placed in the chamber for 30 minutes before experiments to allow them reach a stable state.
2.4. Amperometric measurements of serotonin
Continuous amperometry, using the three electrode system describe above, was also employed for 5-HT measurements in the tissue. Data were collected using a multichannel potentiostat (BioStat, Bioscience, Inc., Chelmsford, MA). The diamond microelectrode was mounted on a micromanipulator (Mitutoyo, Co., Aurora, IL) for precise positioning of the electrode. The microelectrode was held at 0.6 V vs. Ag|AgCl reference electrode PROOF for detection. At this potential, serotonin is oxidized at a mass transfer limited rate.
3. Results and Discussion
3.1. Detection of serotonin using BDD microelectrodes
Differential pulse voltammetry and a BDD microelectrode were used to measure oxidation currents in standard solutions of serotonin mixed in Krebs’ buffer. A well defined oxidation peak for serotonin is apparent at 0.4 V, as shown in Fig. 1. In in vitro measurements, it is necessary to verify that any pharmacological agents used are not themselves electroactive at the recording microelectrode and that they do not affect the response for the neurosignaling molecule of interest. As shown in the figure, citalopram in Krebs’ solution, which is a well-used SSRI, exhibited no electrochemical activity in the potential region where serotonin is oxidized. Furthermore, exposure to the drug had no effect on the serotonin oxidation current as a measurement made following drug exposure and washout produced an identical oxidation peak in term of both potential (~0.4 V) and current (~0.7 nA).
Fig. 1.
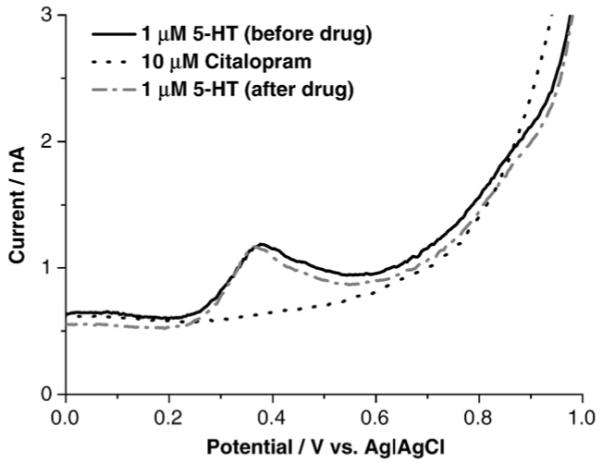
Differential pulse voltammetric i–E curves for 10 μM citalopram in Krebs’ buffer (pH=7.4) (dotted line), and 1 μM serotonin in Krebs’ solution before (solid line) and after (dash-dotted line) exposure citalopram.
3.2. Continuous amperometric recording from tissue
Due to the complex chemical and biological nature of the GI tract, one of the challenges in in vitro electrochemical measurements is electrode fouling by irreversibly adsorbed proteins, lipids and other biomolecules. These molecules can adsorb onto the electrode and block sites involved in the serotonin oxidation reaction, thus lowering oxidation currents (i.e., reduced sensitivity). Additionally, serotonin oxidation reaction products tenaciously adsorb on carbon fiber microelectrodes rapidly blocking the electrode from further reaction [19]. A great advantage of the BDD electrodes is their resistance to molecular adsorption and fouling, as well as resistance to fouling by serotonin oxidation reaction products [9,20]. In this study, a BDD microelectrode was typically employed in tissue for multiple hours without significant serotonin oxidation current attenuation (Fig. 2). The distance of the recording microelectrode from the release sites is critical for these measurements. In an attempt to reproducibly control this distance, we first positioned the BDD microelectrode against the surface of the tissue. This represented the zero-distance point. This step is somewhat subjective depending on the to the researcher’s experience, as a variation of up to ±10 μm can exist between measurements. Next, the BDD microelectrode was withdrawn from the tissue to a fixed distance using the micromanipulator. Prior to positioning near the tissue, the BDD microelectrode was introduced to the perfusing Krebs’ buffer in the tissue chamber at a distance far from the tissue (2 mm). The shear stress of the flowing solution activates the EC cells to release serotonin, but at a far distance, the local serotonin concentration is too low to be oxidatively detected. Only a background current of 0.1 to 0.4 nA is observed. The microelectrode was then positioned near the release sites at a distance of 250 μm. At this distance, the 5-HT concentration is much higher and a corresponding oxidation is recorded. It can be seen that the current increased to about 0.8 nA and remained fairly stable at 0.5 to 1.0 nA level over the 100 minutes period. At the end of this period, the microelectrode was withdrawn from the tissue and the oxidation current decreased to the background level of about 0.1 nA.
Fig. 2.
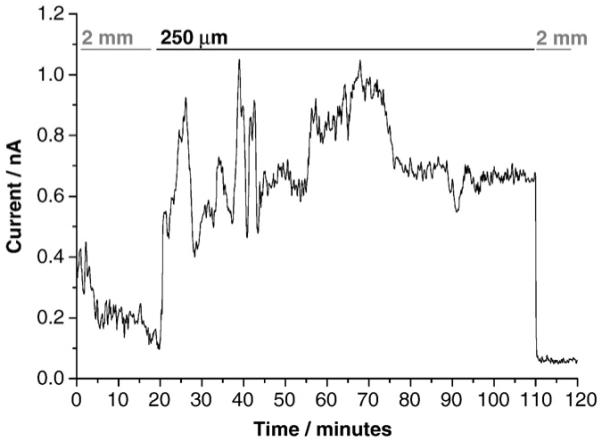
Continuous amperometric measurement of 5-HT released from enterochromaffin cells in the intestinal mucosa at a detection potential of 0.6 V vs. Ag|AgCl. Measurements were made with the microelectrode positioned far (2 mm), close (250 μm), and far (2 mm) again from the tissue. Krebs’ buffer was continuously perfused through the flow bath.
To restate the point, the position of the microelectrode in these measurements strongly affects the measured current. To study this, a serotonin oxidation current was recorded amperometrically as a function of distance from the mucosa (Fig. 3). In this study, the BDD microelectrode was positioned progressively closer to the tissue, initially with a step of 0.5 mm followed by steps of 0.25 mm (Fig. 3a and b). At each distance, a steady-state serotonin oxidation current (Fig. 3a) was recorded. This indicates the electrode provides a stable response for this neurotransmitter. Furthermore, the same steady-state currents were obtained before and after exposure to citalopram (1 μM). This confirms that the drug causes no response attenuation for serotonin. In order to minimize the response variation in tissue, three measurements were averaged at each distance before and after the drug application in five test animals. It was found that the serotonin oxidation current increases significantly (P<0.05, T-test, n=5) in the presence of citalopram (1 μM) when the microelectrode was positioned at a medium distance (0.5 to 1 mm) from the tissue (Fig. 3b). When the microelectrode was gently touching the tissue (at zero-distance point), the oxidation current was greatest. Obviously, the released serotonin concentration is highest near the tissue but also the mechanical touching stimulates the EC cells and thus causes extra serotonin release. In contrast, with the microelectrode far away from the tissue, the serotonin concentration is too low to be detected so there is no measured effect of the SERT-blocking drug. Only when the microelectrode was close enough to the tissue but not physically contacting it, was the serotonin oxidation current sensitive to the SERT blocker. Indeed, the serotonin oxidation current increased 30 to 50% in the presence of citalopram (1 μM) when the microelectrode is positioned at a medium distance (0.5 to 1 mm) from the tissue. This result is consistent with the reports from other investigators [21]. The oxidation current increased with SERT blockade because the extracellular concentration of serotonin increases due to less efficient reuptake.
Fig. 3.
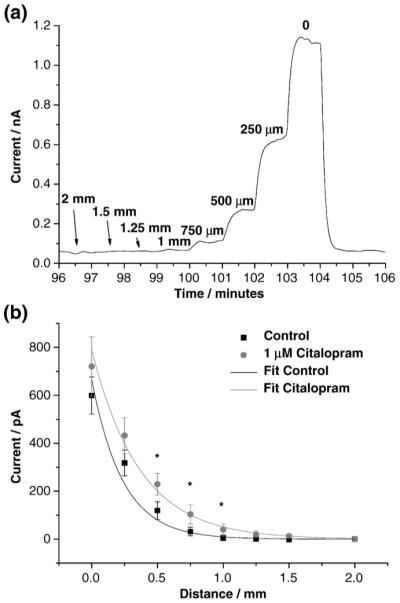
Continuous amperometric measurement of 5-HT released from enterochromaffin cells in the intestinal mucosa at a detection potential of 0.6 V vs. Ag|AgCl. (a) An original training of oxidation current measured over 10 minutes as an example that the oxidation current is a function of how close the microelectrode is positioned to the tissue. (b) The distance-current relationship of 5-HT detection in vitro in the absence and presence of the SERT blocker, citalopram (1 μM) (n=5).
4. Conclusions
The results presented demonstrate that BDD microelectrodes can be used to stably measure serotonin availability in vitro in the ileum mucosa of the guinea pig, in the absence and presence of the SERT-blocker, citalopram. Due to the absence of an extended π-electron system and a predominately H surface termination, serotonin oxidation reaction products do not strongly adsorb onto the diamond surface; a process that is pervasive on sp [2] carbon electrodes and produced near total oxidation current attenuation for serotonin due to fouling. Furthermore, the results demonstrate how important the electrode-tissue distance is in for sensitively measuring pharmacologically-manipulated serotonin release from EC cells.
Acknowledgements
This work was generously supported by grants from the National Institutes of Health, HD056197 (X. B.), DK57039 (J. J. G.) and DK082867 (G. M. S.).
References
- [1].Swain GM, Ramesham R. Anal. Chem. 1993;65:345. [Google Scholar]
- [2].Swain GM. Adv. Mater. 1994;6:388. [Google Scholar]
- [3].Park J, Quaiserova-Mocko V, Patel BA, Novotny M, Liu AH, Bian XC, Galligan JJ, Swain GM. Analyst. 2008;133:17. doi: 10.1039/b710236b. [DOI] [PubMed] [Google Scholar]
- [4].Park J, Galligan JJ, Fink GD, Swain GM. Anal. Chem. 2006;78:6756. doi: 10.1021/ac060440u. [DOI] [PubMed] [Google Scholar]
- [5].Halpern JM, Xie ST, Sutton GP, Higashikubo BT, Chestek CA, Lu H, Chiel HJ, Martin HB. Diam. Relat. Mater. 2006;15:183. [Google Scholar]
- [6].Suzuki A, Ivandini TA, Yoshimi K, Fujishima A, Oyama G, Nakazato T, Hattori N, Kitazawa S, Einaga Y. Anal. Chem. 2007;79:8608. doi: 10.1021/ac071519h. [DOI] [PubMed] [Google Scholar]
- [7].Cvacka J, Quaiserova V, Park J, Show Y, Muck A, Swain GM. Anal. Chem. 2003;75:2678. doi: 10.1021/ac030024z. [DOI] [PubMed] [Google Scholar]
- [8].Park J, Quaiserova-Mocko V, Peckova K, Galligan JJ, Fink GD, Swain GM. Diam. Relat. Mater. 2006;15:761. [Google Scholar]
- [9].Patel BA, Bian XH, Quaiserova-Mocko V, Galligan JJ, Swain GM. Analyst. 2007;132:41. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- [10].Hansen MB, Witte AB. Acta Physiol. 2008;193:311. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- [11].Mawe GM, Coates MD, Moses PL. Aliment. Pharmacol. Ther. 2006;23:1067. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- [12].Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. J. Neurosci. 1996;16:2352. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gershon MD. Aliment. Pharmacol. Ther. 2004;20:3. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- [14].Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Gastroenterology. 2004;126:1657. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [15].Spiller R. Neuropharmacology. 2008;55:1072. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- [16].Miwa J, Echizen H, Matsueda K, Umeda N. Digestion. 2001;63:188. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- [17].Zhao H, O’Hare D. J. Phys. Chem. C. 2008;112:9351. [Google Scholar]
- [18].Ranganathan S, Kuo TC, McCreery RL. Anal. Chem. 1999;71:3574. [Google Scholar]
- [19].Jackson BP, Dietz SM, Wightman RM. Anal. Chem. 1995;67:1115. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- [20].Bian XC, Patel B, Dai XL, Galligan JJ, Swain G. Gastroenterology. 2007;132:2438. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bertrand PP, Hu X, Mach J, Bertrand RL. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1228. doi: 10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]


