Abstract
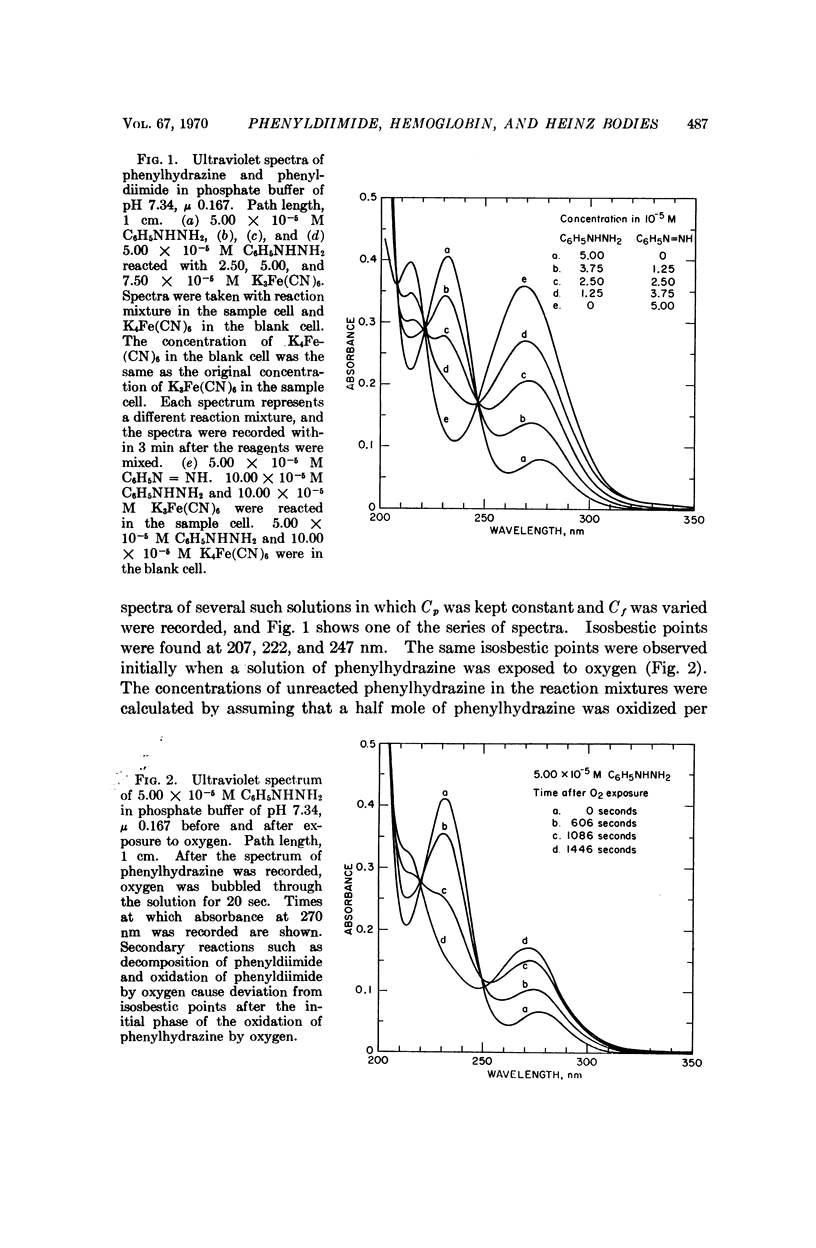
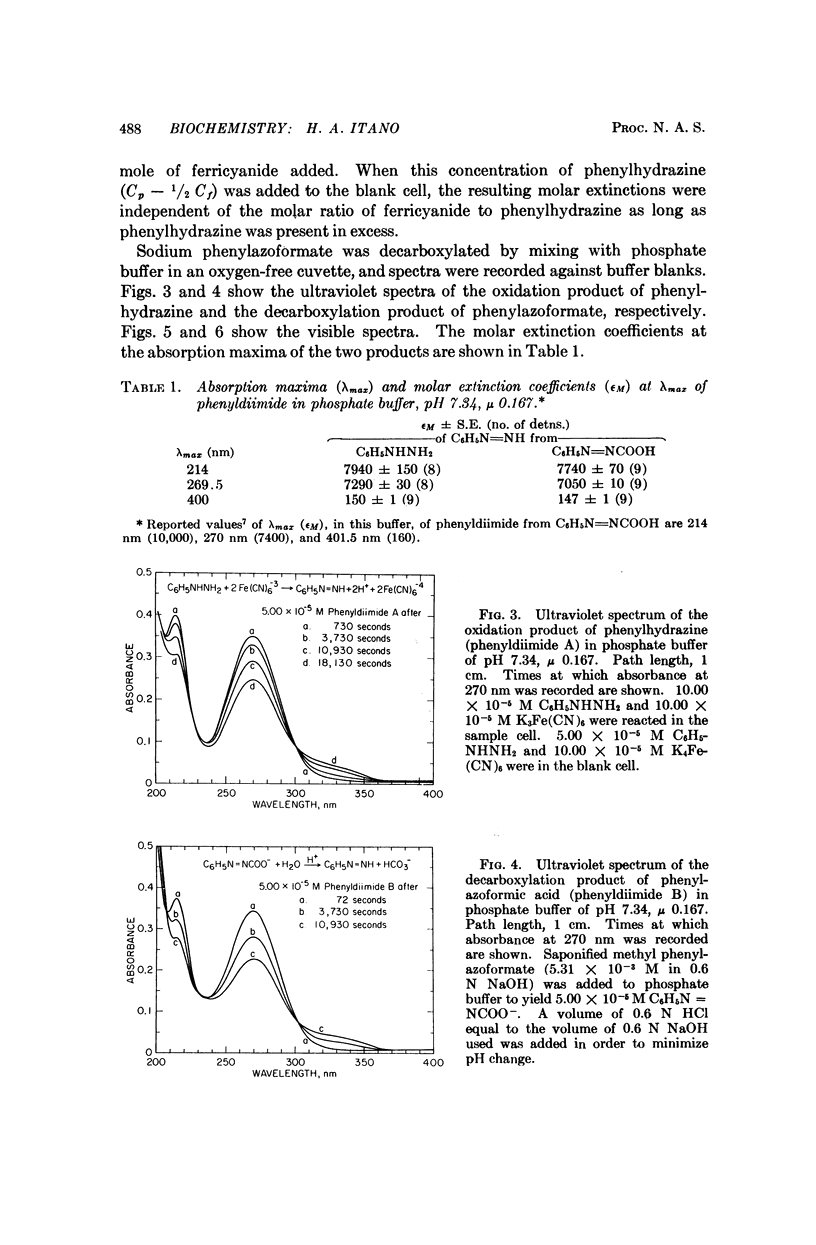
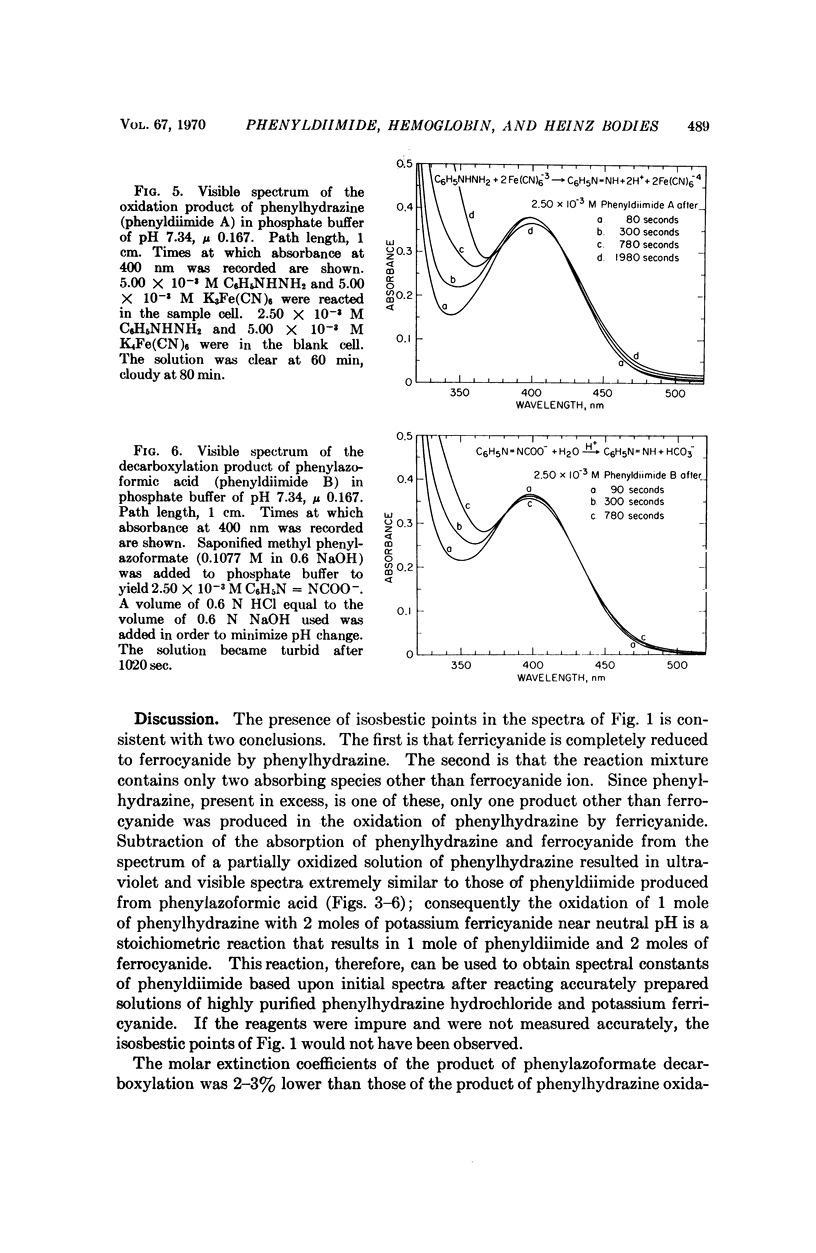
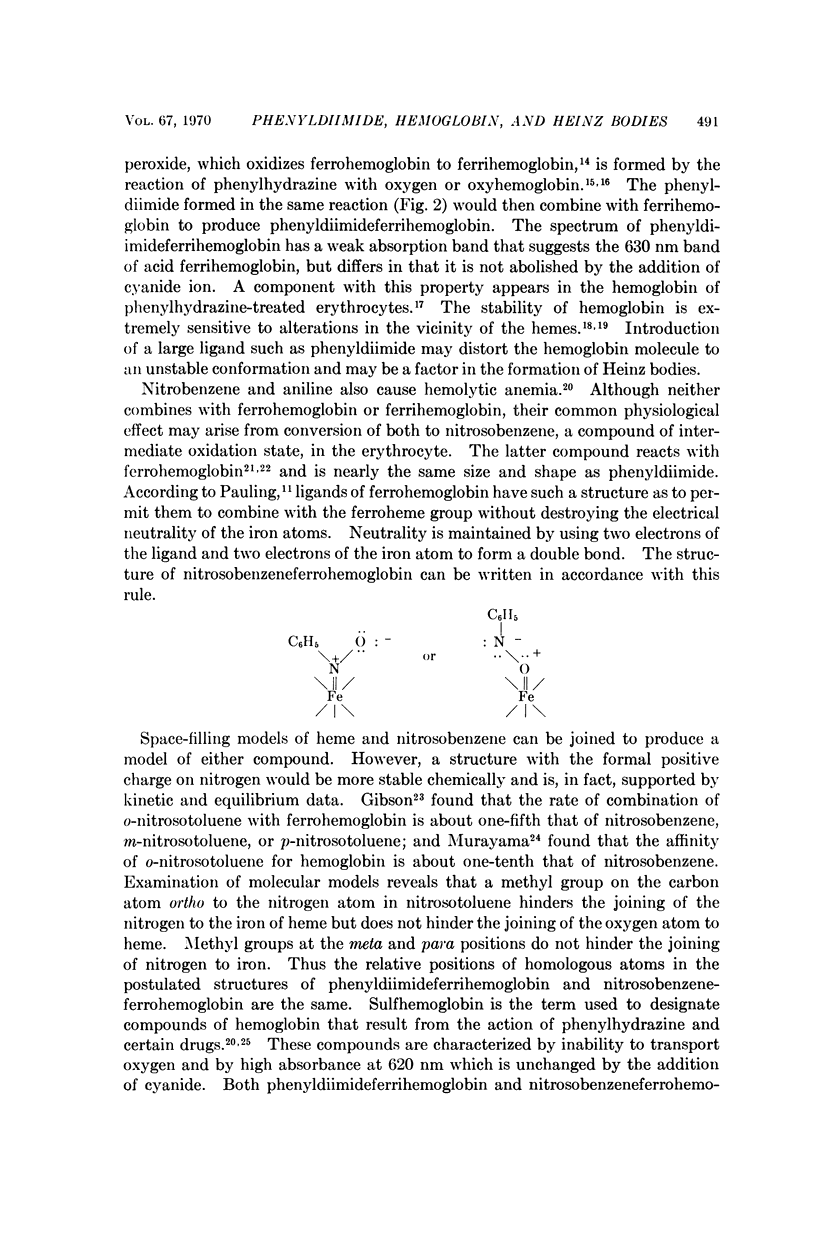
Phenylhydrazine is oxidized stoichiometrically by two equivalents of ferricyanide to produce phenyldiimide and ferrocyanide. This reaction permits accurate determination of the absorption maxima and molar absorption coefficients of phenyldiimide in aqueous solution. These values are identical or extremely similar to those of phenyldiimide produced by decarboxylation of phenylazoformic acid. Knowledge of the stoichiometry of the reaction confirms an earlier suggestion that phenyldiimide is the product of ferricyanide-oxidized phenylhydrazine that reacts with heme proteins. Homologous structures postulated for phenyldiimideferrihemoglobin and nitrosobenzeneferrohemoglobin suggest a common factor in the production of Heinz bodies after exposure of erythrocytes to phenylhydrazine and to arylamines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H. The reactions of some aromatic C-nitroso compounds with haemoglobin. Biochem J. 1960 Dec;77:519–526. doi: 10.1042/bj0770519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAAN J., KIESE M., WERNER A. Reduktion von Nitrosobenzol zu Anilin in roten Blutzellen. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;235(4):365–372. [PubMed] [Google Scholar]
- Hanstein W. G., Lett J. B., McKenna C. E., Traylor T. G. Heme protein-diimide complex- es: possible intermediates in biological nitrogen fixation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1314–1316. doi: 10.1073/pnas.58.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc Natl Acad Sci U S A. 1970 Mar;65(3):697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIESE M. Oxydation von Anilin zu Nitrosobenzol im Hunde. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;235(4):354–359. [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- MURAYAMA M. The combining power of normal human hemoglobin for nitrosobenzene. J Biol Chem. 1960 Apr;235:1024–1028. [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- ROSTORFER H. H., CORMIER M. J. The formation of hydrogen peroxide in the reaction of oxyhemoglobin with methemoglobin-forming agents. Arch Biochem Biophys. 1957 Sep;71(1):235–249. doi: 10.1016/0003-9861(57)90025-5. [DOI] [PubMed] [Google Scholar]
- ROSTORFER H. H., TOTTER J. R. The reduction of methemoglobin by phenylhydrazine under anaerobic conditions. J Biol Chem. 1956 Aug;221(2):1047–1055. [PubMed] [Google Scholar]


