Abstract
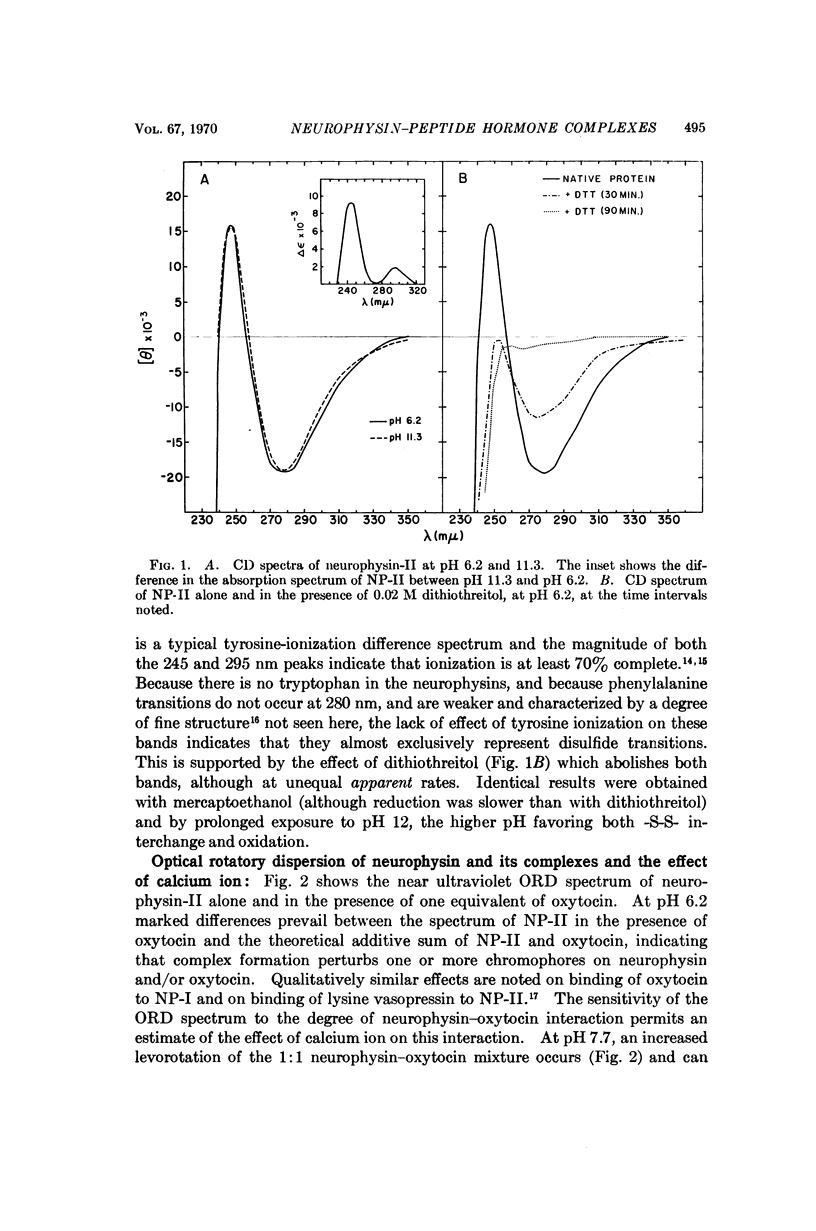
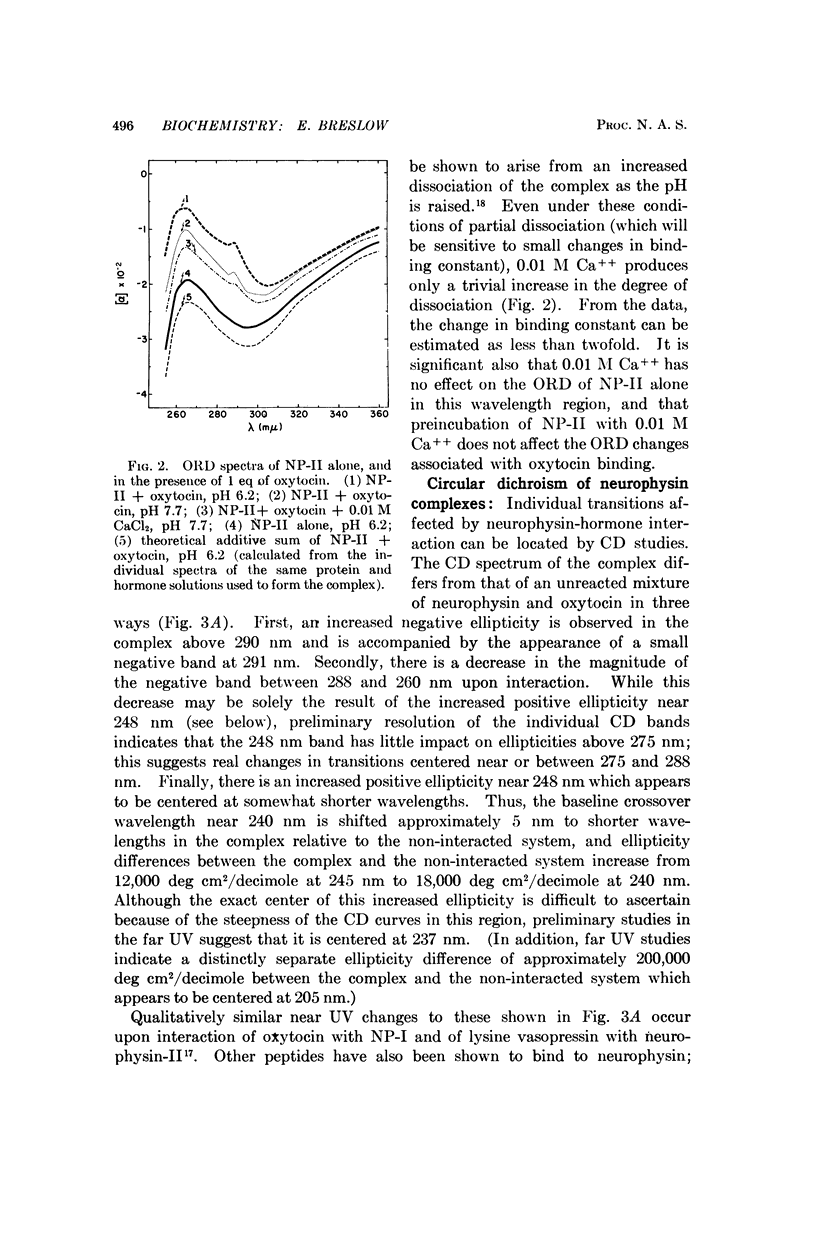
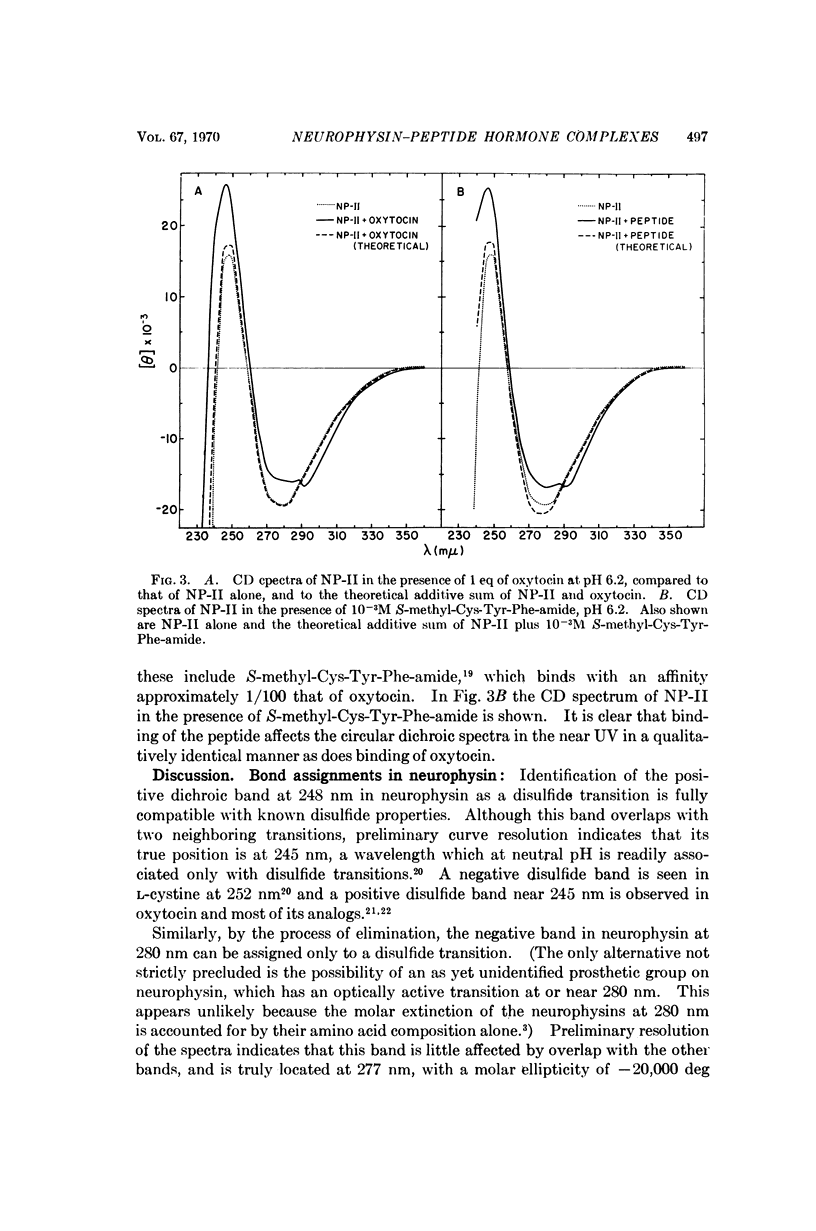
Circular dichroism studies of the bovine neurophysins in the near ultraviolet show a strong negative band at 280 nm and a strong positive band at 248 nm, both of which are attributable almost exclusively to disulfide transitions. The ellipticities per disulfide bond of the unresolved bands in neurophysin-II are -2900 deg cm2/decimole and +2300 deg cm2/decimole at 280 nm and 248 nm, respectively. Binding of oxytocin, vasopressin, or the peptide S-methyl-L-cysteinyl-L-tyrosyl-L-phenylalanine amide lead to large changes in optical activity in the near and far ultraviolet. Of these circular dichroism changes above 290 nm are attributed to changes in the optical activity of neurophysin disulfides, while changes elsewhere are more generally ascribed to changes in either disulfide, tyrosine, or peptide bond transitions. Optical rotatory dispersion studies show that calcium ion, at concentrations of 0.01 M, has only trivial effects on the affinity of bovine neurophysins for oxytocin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW E. The cupric ion complexes of oxytocin and 2-phenylalanine oxytocin. Biochim Biophys Acta. 1961 Nov 11;53:606–609. doi: 10.1016/0006-3002(61)90230-x. [DOI] [PubMed] [Google Scholar]
- Beychok S., Breslow E. Circular dichroism of oxytocin and several oxytocin analogues. J Biol Chem. 1968 Jan 10;243(1):151–154. [PubMed] [Google Scholar]
- Beychok S. Circular dichroism of biological macromolecules. Science. 1966 Dec 9;154(3754):1288–1299. doi: 10.1126/science.154.3754.1288. [DOI] [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth A. J., Hope D. B. Studies on the chemical modification of the tyrosine residue in bovine neurophysin-II. Biochem J. 1970 Feb;116(4):545–553. doi: 10.1042/bj1160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. BINDING OF VASOPRESSIN AND OXYTOCIN TO PROTEIN IN EXTRACTS OF BOVINE AND RABBIT NEUROHYPOPHYSES. J Endocrinol. 1964 Aug;30:131–145. doi: 10.1677/joe.0.0300131. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Jayasena K., Thomas P. J. The preparation and properties of porcine neurophysin and the influence of calcium on the hormone-neurophysin complex. J Physiol. 1966 May;184(2):387–401. doi: 10.1113/jphysiol.1966.sp007921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZWARTH G., DOTY P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965 Jan 20;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. Fractionation of neurophysin by molecular-sieve and ion-exchange chromatography. Biochem J. 1967 Jul;104(1):122–127. doi: 10.1042/bj1040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J., Strickland E. H., Billups C. Analysis of the vibrational structure in the near-ultraviolet circular dichroism and absorption spectra of tyrosine derivatives and ribonuclease-A at 77 degrees K. J Am Chem Soc. 1970 Apr 8;92(7):2119–2129. doi: 10.1021/ja00710a054. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Strickland E. H., Billups C. Analysis of vibrational structure in the near-ultraviolet circular dichroism and absorption spectra of phenylalanine and its derivatives. J Am Chem Soc. 1969 Jan 1;91(1):184–190. doi: 10.1021/ja01029a034. [DOI] [PubMed] [Google Scholar]
- Rauch R., Hollenberg M. D., Hope D. B. Isolation of a third bovine neurophysin. Biochem J. 1969 Nov;115(3):473–479. doi: 10.1042/bj1150473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M. W., THORN N. A. THE EFFECTS OF CALCIUM ON PROTEIN-BINDING AND METABOLISM OF ARGININE VASOPRESSIN IN RATS. J Endocrinol. 1965 May;32:141–151. doi: 10.1677/joe.0.0320141. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Quadrifoglio F., Walter R., Schwartz I. L. Conformational studies on neurohypophyseal hormones: the disulfide bridge of oxytocin. Proc Natl Acad Sci U S A. 1968 Jul;60(3):967–974. doi: 10.1073/pnas.60.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuu T. C., Saffran M. Isolation and characterization of a hormone-binding polypeptide from pig posterior pituitary powder. J Biol Chem. 1969 Jan 25;244(2):482–490. [PubMed] [Google Scholar]


