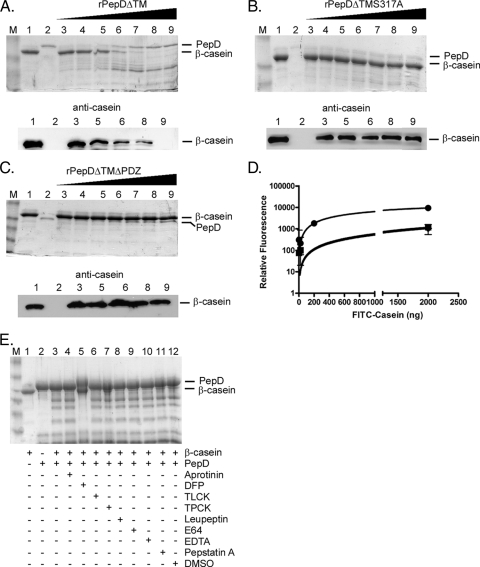
FIG. 1.
rPepDΔTM exhibits proteolytic activity against the artificial substrate β-casein. Protease assays were carried out with the artificial substrate β-casein and rPepDΔTM (A), rPepDΔTMS317A (B), or rPepDΔTMΔPDZ (C) for 16 h at 37°C. The reaction mixtures contained 1 μg β-casein alone (lanes 1), 500 ng rPepD variant alone (lanes 2), or 1 μg β-casein incubated with increasing concentrations (67 to 500 ng) of rPepD variants (lanes 3 to 9). The reaction products were resolved by SDS-PAGE and visualized by either Coomassie staining or Western blotting using a polyclonal antibody against casein. (D) The cleavage of β-casein by rPepD variants was quantified using 41.5 μg FITC-labeled casein as a substrate and measuring the relative fluorescence of reaction products (loss of FITC quenching) as a function of the rPepD concentration. The rPepDΔTMS317A and rPepDΔTMΔPDZ variants exhibited approximately 10% of the activity of rPepDΔTM. •, rPepDΔTM; ▪, rPepDΔTMS317A; ⧫, rPepDΔTMΔPDZ. The error bars indicate standard errors. (E) The specificity of rPepDΔTM-mediated β-casein cleavage was determined by the inclusion of various protease inhibitors in the reaction mixture. The reaction mixtures contained 1 μg β-casein alone (lane 1), 500 ng rPepDΔTM alone (lane 2), or 1 μg β-casein and 500 ng rPepDΔTM (lane 3) incubated with 1.5 mM aprotinin (lane 4), 5 mM DFP (lane 5), 27 mM TLCK (lane 6), 28 mM TPCK (lane 7), 23 mM leupeptin (lane 8), 1 mM E64 (lane 9), 50 mM EDTA (lane 10), 1 mM pepstatin A (lane 11), or DMSO (lane 12). Inhibition was observed in the reaction mixture containing DFP. Lanes M, molecular weight marker.