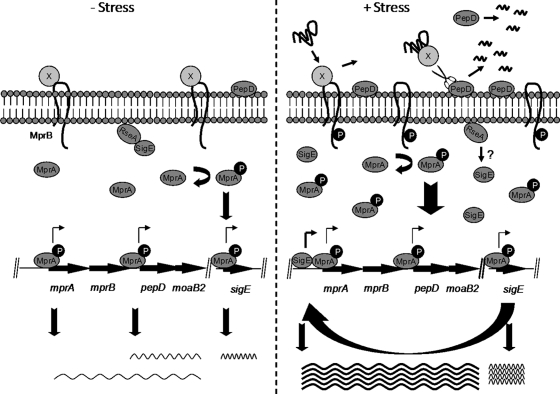
FIG. 7.
Model of the activation of the MprAB system. Under nonstress conditions (left), an unidentified protein repressor (protein X) binds to MprB, keeping it in an inactive conformation and resulting in basal-level expression of mprAB transcription. In the presence of membrane stress (right), misfolded proteins accumulate in the extracytoplasmic space, resulting in the release of protein X from MprB and activation of the MprAB signaling pathway, including upregulation of sigE and subsequent induction of mprAB-pepD-moaB. PepD is secreted into the extracytoplasmic space between the cell membrane and the cell wall, where it functions to degrade and/or refold damaged substrate targets. Following proteolysis, the PDZ domain of PepD is removed in an autocatalytic event, resulting in release of PepD into the culture supernatant. Wavy lines indicate transcripts, with darker lines indicating higher expression lavels.