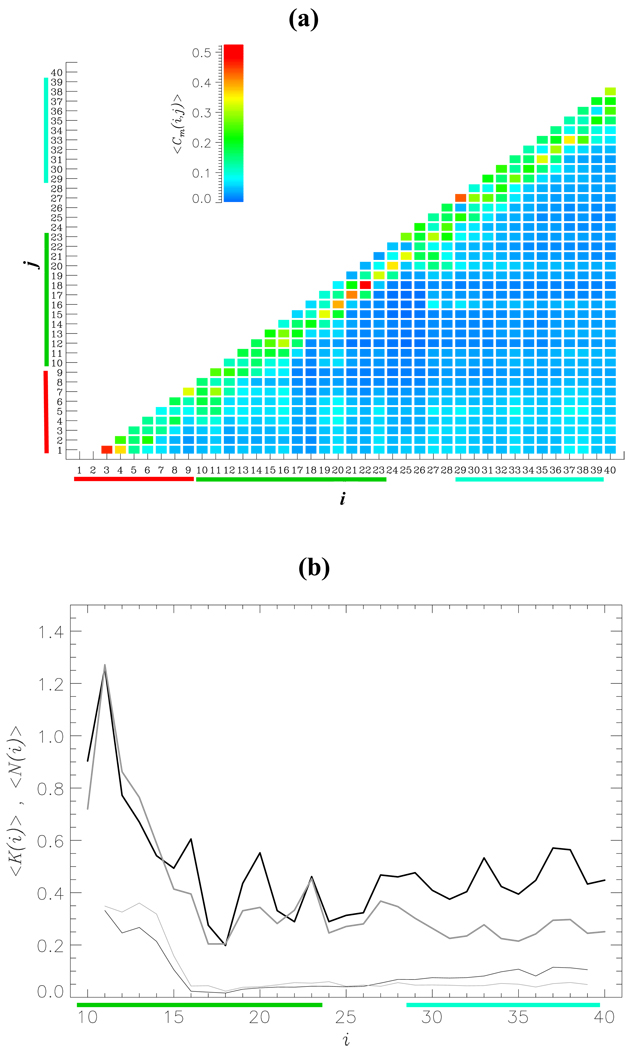
Fig. 5.
(a) The thermal contact map < Cm(i, j) > displays the probabilities of forming side chain contacts between amino acids i and j in Aβ1–40 monomer. < Cm(i, j) > is obtained by averaging over the canonical ensemble generated by REMD and is color coded according to the scale shown. The contact map indicates that the NT region forms interactions with the CR and CT regions. (b) The numbers of intrapeptide side chain contacts < K(i) > and HBs < N(i) > formed by the NT with the residues i (10 ≤ i ≤ 40) are shown by thick and thin curves, respectively. The data for monomer and dimer are in black and gray. Compared to side chain contacts the NT region forms few HBs with the rest of Aβ1–40 sequence.