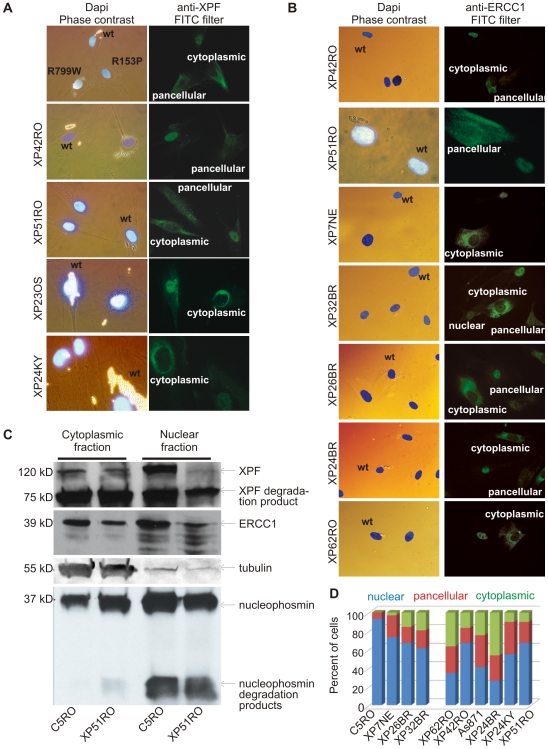
Figure 2. Differential immunofluorescence of cells from patients with XPF mutations.
Fibroblasts from patients with mutations in XPF and a normal control were grown in the presence of different size beads. After 24 hr the cultures were washed to remove extracellular beads, mixed and co-plated on glass coverslips. The next day, the cells were fixed and immunostained as indicated. Cells were stained with Dapi to identify nuclei and examined by phase contrast microscopy to identify the cell type by their bead content and by fluorescence microscopy for immunodetection of XPF or ERCC1. (A) Analysis of XPF protein sub-cellular localization. Cells from an unaffected individual were labeled with 2 µM beads; XPF mutant cells were labeled with 0.8 µM beads. (B) Analysis of ERCC1 subcellular localization in patients with mutations in XPF. (C) Immunodetection of XPF and ERCC1 in nuclear and cytoplasmic fractions of normal fibroblasts (C5RO) and XPF mutant cells (XP51RO). Tubulin is used as a loading control of the cytoplasmic fraction. Nucleophosmin is used as a loading control for the nuclear fraction. (D) Quantitation of the fraction of cells containing exclusively nuclear XPF-ERCC1, XPF-ERCC1 in the nucleus and cytoplasm (pancellular) or exclusively cytoplasmic complex, as determined from immunofluorescence images (n≥100 cells per cell line).