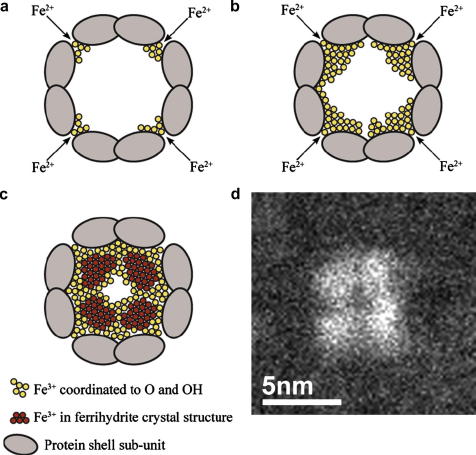
Fig. 4.
Schematic cross-section (viewing direction: parallel to one of the four-fold symmetry channels in the protein shell) of a hepatic ferritin core depicting our proposed formation mechanism. This is a modification of a schematic of core formation by Lewin et al. (2005). (a) Early stage of iron deposition in the ferritin central cavity. The sites near the ends of the three-fold symmetry iron entry channels (where the protein shell subunits, shown as grey lobes, have specific oxidation sites) are favourable for the incoming Fe2+ to deposit and be oxidised. The yellow circles represent oxidised iron (Fe3+). (b) As the iron cellular concentration increases, more Fe2+ is shuffled into the molecule and may rapidly deposit and oxidise on the surface of any existing Fe3+ deposits near the entry channels; consequently, core subunits are formed. (c) With higher iron-filling, a cubic-like core structure with eight subunits (only four of which are shown) develops. Oxidation of further incoming Fe2+, results in the early deposited Fe3+ diffusing inwards forming closely packed crystalline structures of ferrihydrite (dark red circles in contrast to the loosely packed Fe3+ (yellow circles)), the atomic structure of such a subunit structure is seen experimentally in Fig. 2d. The surface of each core subunit is disordered facilitating dynamic load and release activities consistent with the ‘last-in first-out’ hypothesis (Hoy et al., 1974). (d) An example of a commonly observed HAADF image of a single ferritin core of similar iron loading and lying in a similar orientation to the schematic; the four-fold symmetry arrangement of the subunits and a low density central region are clearly evident.