Abstract
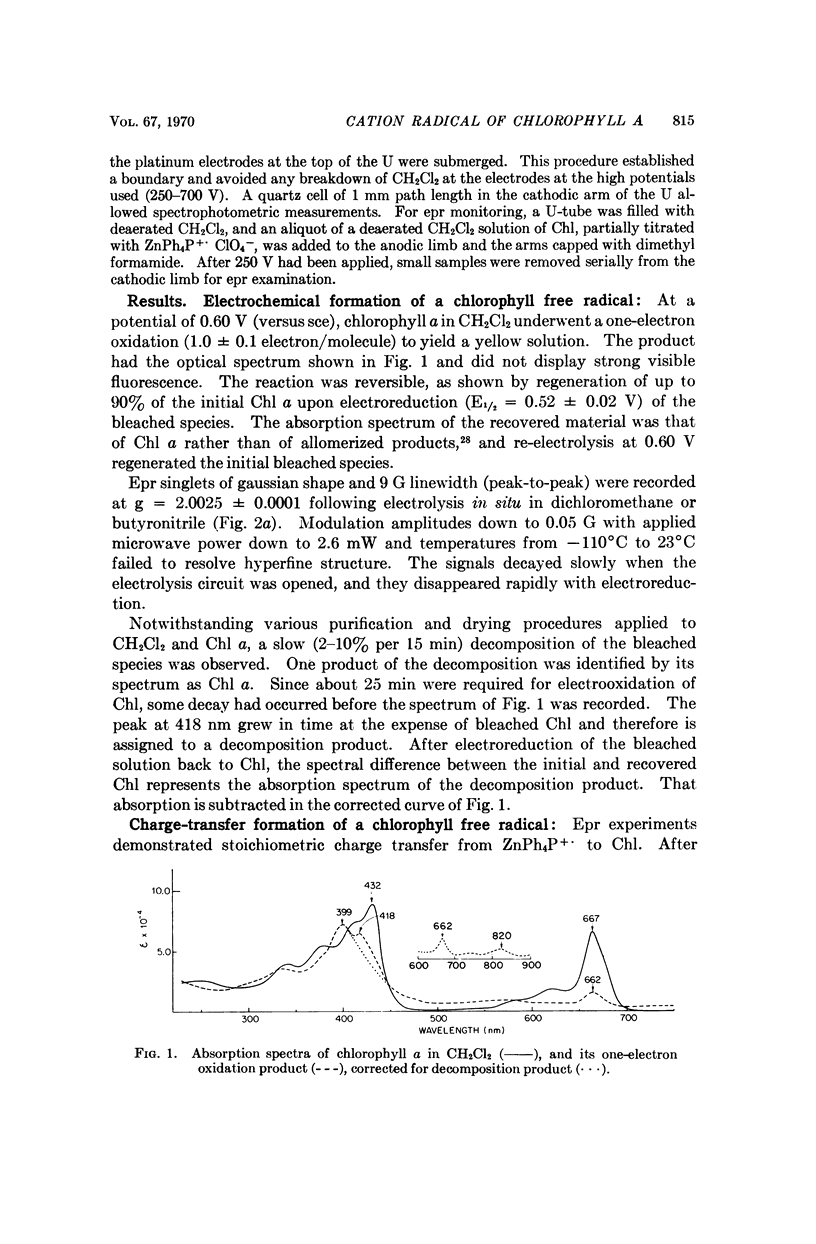
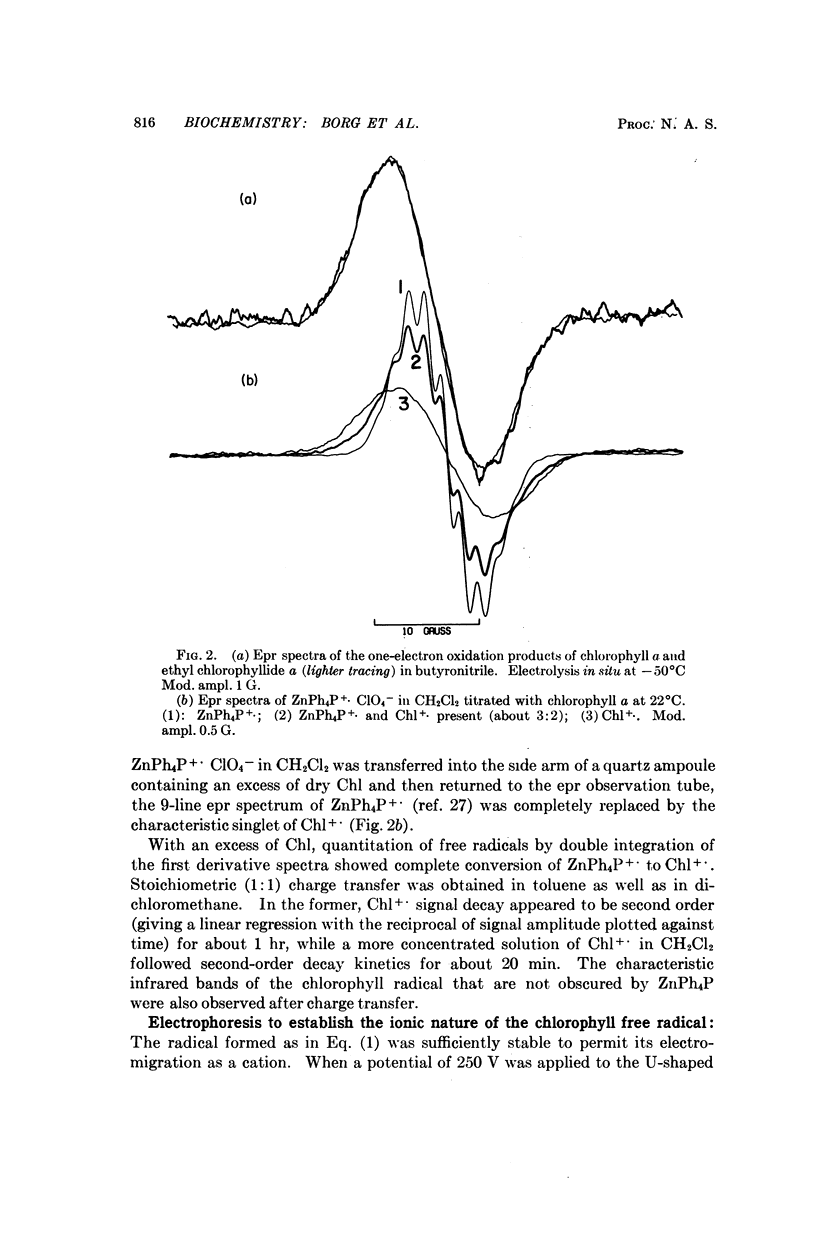
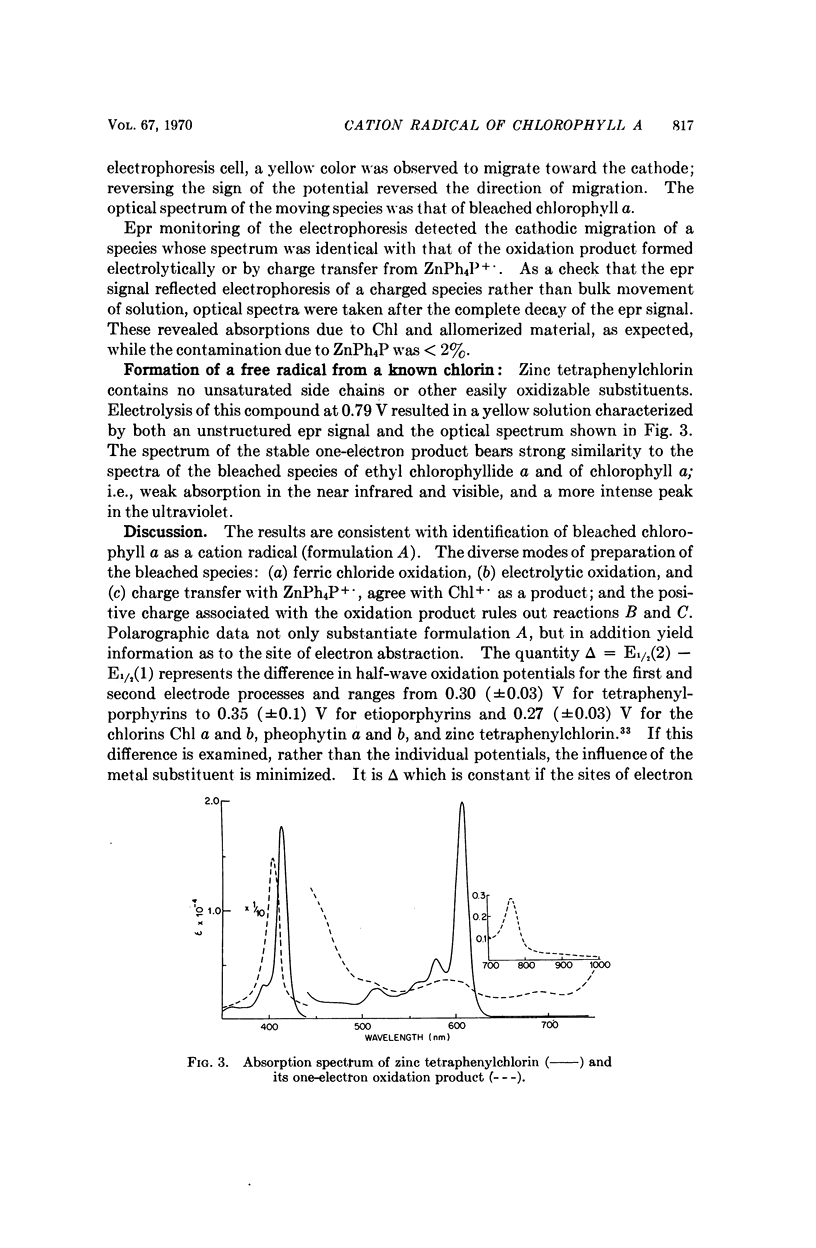
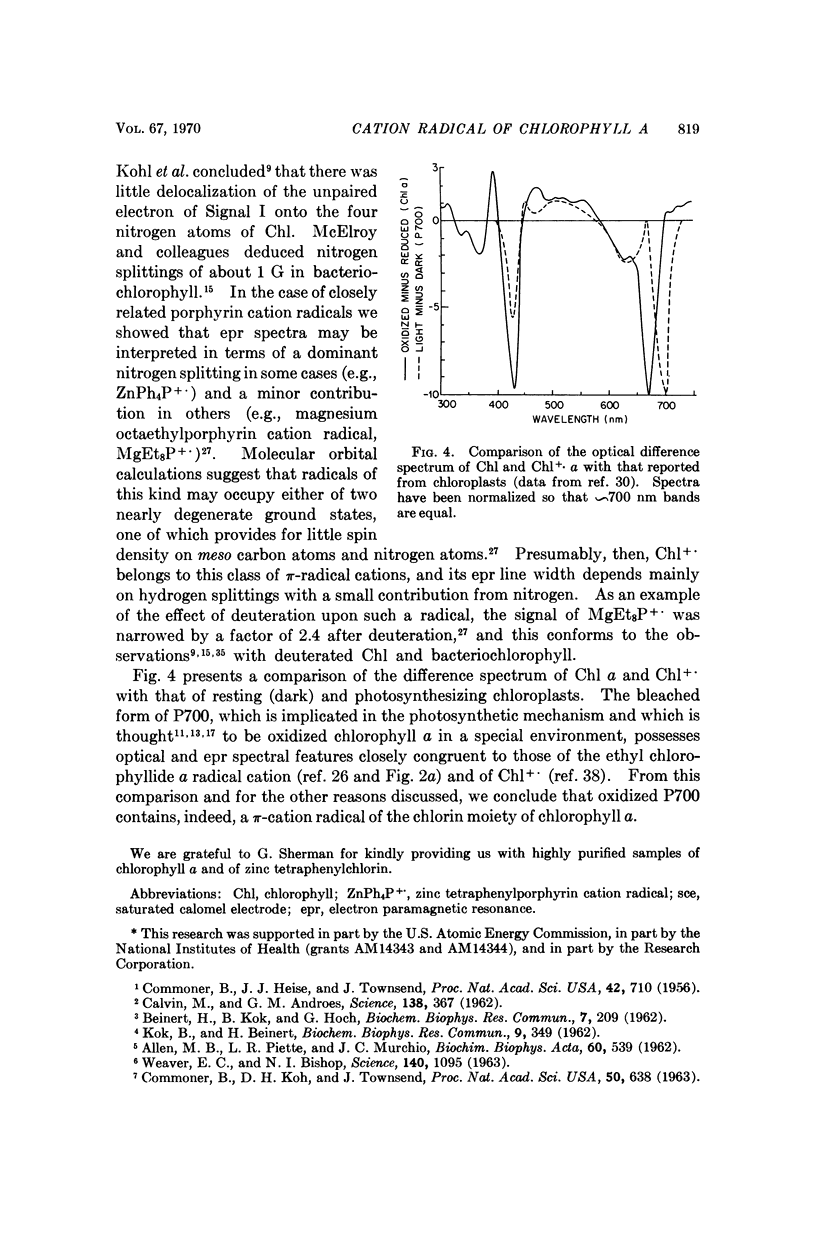
Chlorophyll a undergoes reversible one-electron oxidation in dichloromethane and butyronitrile. Removal of the electron by controlled potential electrolysis or by stoichiometric charge transfer to a known cation radical yields a radical (epr line width = 9 gauss, g = 2.0025 ± 0.0001) whose optical spectrum is bleached relative to that of chlorophyll. Upon electrophoresis this bleached species behaves as a cation. By comparison with the known properties of π-cation radicals of porphyrins and chlorins, the chlorophyll radical is also identified as a π-cation. Further correlation of optical and epr properties with published studies on photosynthesis leads to the conclusion that oxidized P700, the first photochemical product of photosystem I in green plants, contains a π-cation radical of the chlorin component of chlorophyll a. This radical is the likely source of the rapidly-decaying, narrow epr signal of photosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B., PIETTE L. R., MURCHIO J. C. Free radicals in photosynthetic reactions. L Electron paramagnetic resonance signals from illuminated Chlorella pyrenoidosa. Biochim Biophys Acta. 1962 Jul 16;60:539–547. doi: 10.1016/0006-3002(62)90872-7. [DOI] [PubMed] [Google Scholar]
- Androes G. M., Singleton M. F., Calvin M. EPR IN CHROMATOPHORES FROM RHODOSPIRILLUM RUBRUM AND IN QUANTASOMES FROM SPINACH CHLOROPLASTS. Proc Natl Acad Sci U S A. 1962 Jun;48(6):1022–1031. doi: 10.1073/pnas.48.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEINERT H., KOK B. AN ATTEMPT AT QUANTITATION OF THE SHARP LIGHT-INDUCED ELECTRON PARAMAGNETIC RESONANCE SIGNAL IN PHOTOSYNTHETIC MATERIALS. Biochim Biophys Acta. 1964 Sep 25;88:278–288. doi: 10.1016/0926-6577(64)90183-4. [DOI] [PubMed] [Google Scholar]
- BEINERT H., KOK B., HOCH G. The light induced electron paramagnetic resonance signal of photocatalyst P700. Biochem Biophys Res Commun. 1962 Apr 20;7:209–212. doi: 10.1016/0006-291x(62)90176-6. [DOI] [PubMed] [Google Scholar]
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- Bolton J. R., Cost K., Frenkel A. W. A kinetic study of the production of light-induced ESR signals in Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1968 Aug;126(2):383–387. doi: 10.1016/0003-9861(68)90421-9. [DOI] [PubMed] [Google Scholar]
- COMMONER B., KOHL D. H., TOWNSEND J. FAST KINETICS OF UNPAIRED ELECTRONS IN PHOTOSYNTHETIC SYSTEMS. Proc Natl Acad Sci U S A. 1963 Oct;50:638–644. doi: 10.1073/pnas.50.4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commoner B., Heise J. J., Townsend J. LIGHT-INDUCED PARAMAGNETISM IN CHLOROPLASTS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):710–718. doi: 10.1073/pnas.42.10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn B., Seely G. R. The oxidation of chlorophyll a in alcohols. Biochim Biophys Acta. 1968 May 28;153(4):862–867. doi: 10.1016/0005-2728(68)90013-3. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Dolphin D., Felton R. H. pi-Cation radicals and dications of metalloporphyrins. J Am Chem Soc. 1970 Jun 3;92(11):3451–3459. doi: 10.1021/ja00714a038. [DOI] [PubMed] [Google Scholar]
- Fuhrhop J. H., Mauzerall D. The one-electron oxidation of metalloporphyrins. J Am Chem Soc. 1969 Jul 16;91(15):4174–4181. doi: 10.1021/ja01043a027. [DOI] [PubMed] [Google Scholar]
- KE B. LIGHT-INDUCED RAPID ABSORPTION CHANGES DURING PHOTOSYNTHESIS. V. DIGITONIN-TREATED CHLOROPLASTS. Biochim Biophys Acta. 1964 Sep 25;88:297–303. doi: 10.1016/0926-6577(64)90185-8. [DOI] [PubMed] [Google Scholar]
- KOK B., BEINERT H. The light induced EPR signal of photocatalyst P700. II. Two light effects. Biochem Biophys Res Commun. 1962 Oct 31;9:349–354. doi: 10.1016/0006-291x(62)90053-0. [DOI] [PubMed] [Google Scholar]
- KOK B. Partial purification and determination of oxidation reduction potential of the photosynthetic chlorophyll complex absorbing at 700 millimicrons. Biochim Biophys Acta. 1961 Apr 15;48:527–533. doi: 10.1016/0006-3002(61)90050-6. [DOI] [PubMed] [Google Scholar]
- Katz J. J., Ballschmiter K., Garcia-Morin M., Strain H. H., Uphaus R. A. Electron paramagnetic resonance of chlorophyll-water aggregates. Proc Natl Acad Sci U S A. 1968 May;60(1):100–107. doi: 10.1073/pnas.60.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl D. H., Townsend J., Commoner B., Crespi H. L., Dougherty R. C., Katz J. J. Effects of isotopic substitution on electron spin resonance signals in photosynthetic organisms. Nature. 1965 Jun 12;206(989):1105–1110. doi: 10.1038/2061105a0. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Walsh K. Quantum yield for the photoproduced electron paramagnetic resonance signal in chromatophores from Rhodospirillum rubrum. Biochemistry. 1969 May;8(5):1908–1913. doi: 10.1021/bi00833a021. [DOI] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]
- RUBY R. H., KUNTZ I. D., Jr, CALVIN M. TRANSIENT EPR AND ABSORBENCE CHANGES IN PHOTPSYNTHETIC BACTERIA. Proc Natl Acad Sci U S A. 1964 Mar;51:515–520. doi: 10.1073/pnas.51.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUMBERG B., WITT H. T. ANALYSE DER PHOTOSYNTHESE MIT BLITZLICHT. I. DIE PHOTOOXYDATION VON CHLOROPHYLL-A1-430-703. Z Naturforsch B. 1964 Aug;19:693–707. [PubMed] [Google Scholar]
- STEINHARDT R. G., Jr, CALVIN A. D., DODD E. A. Taste-structure correlation with alpha-D-mannose and beta-D-mannose. Science. 1962 Feb 2;135(3501):367–368. doi: 10.1126/science.135.3501.367. [DOI] [PubMed] [Google Scholar]
- TOLLIN G., GREEN G. Light-induced single electron transfer reactions between chlorophyll a and quinones in solution. I. Some general feature of kinetics and mechanism. Biochim Biophys Acta. 1962 Jul 16;60:524–538. doi: 10.1016/0006-3002(62)90871-5. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Ke B., Shaw E. R. Relationship of P700, electron spin resonance signal, and photochemical activity of a small chloroplast particle obtained by the action of Triton X-100. Biochemistry. 1967 Jul;6(7):2210–2220. doi: 10.1021/bi00859a044. [DOI] [PubMed] [Google Scholar]
- Weaver E. C., Bishop N. I. Photosynthetic Mutants Separate Electron Paramagnetic Resonance Signals of Scenedesmus. Science. 1963 Jun 7;140(3571):1095–1097. doi: 10.1126/science.140.3571.1095. [DOI] [PubMed] [Google Scholar]
- Weaver E. C. Electron paramagnetic resonance signal produced by ferricyanide in photosynthetic plant materials. Biochim Biophys Acta. 1968 Aug 20;162(2):286–289. doi: 10.1016/0005-2728(68)90110-2. [DOI] [PubMed] [Google Scholar]
- Weaver E. C., Weaver H. E. Paramagnetic unit in spinach subchloroplast particles: estimation of size. Science. 1969 Aug 29;165(3896):906–907. doi: 10.1126/science.165.3896.906. [DOI] [PubMed] [Google Scholar]
- Wolberg A., Manassen J. Electrochemical and electron paramagnetic resonance studies of metalloporphyrins and their electrochemical oxidation products. J Am Chem Soc. 1970 May 20;92(10):2982–2991. doi: 10.1021/ja00713a010. [DOI] [PubMed] [Google Scholar]


