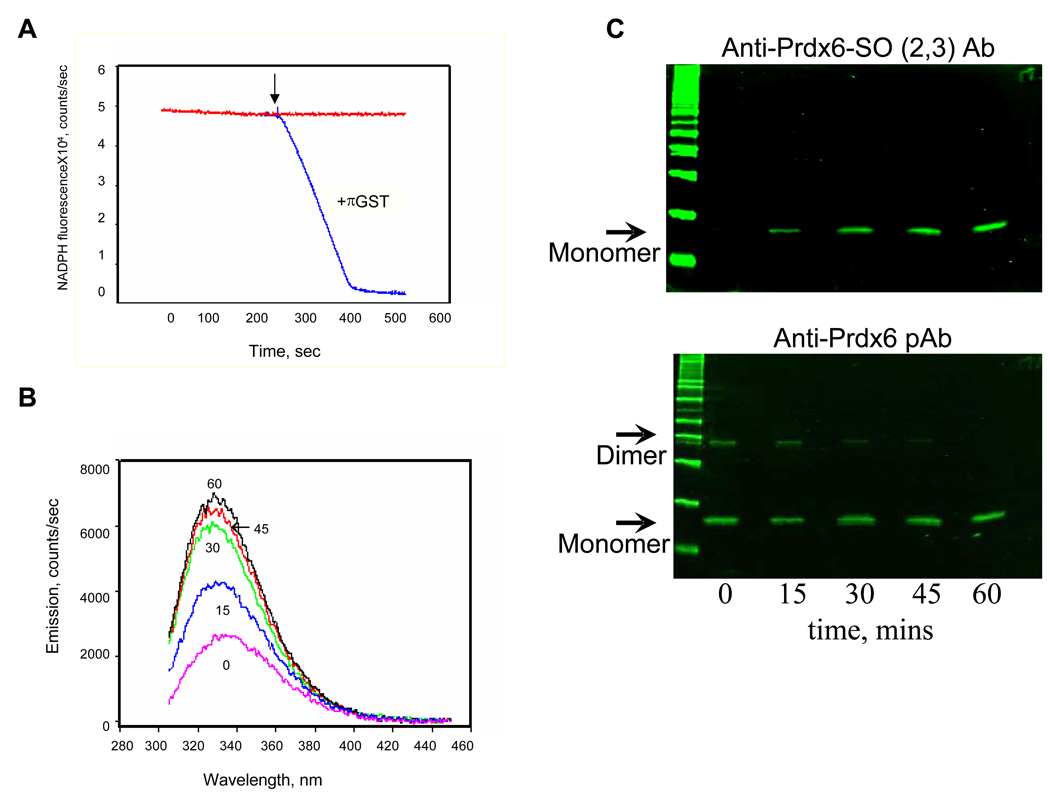
Figure 1. Prdx6 peroxidase activity with phospholipid hydroperoxide (PLPCOOH) substrate.
A. Peroxidase activity measured by the change in NADPH fluorescence in a coupled assay in the presence of GSH and GSH reductase [7]. The reaction was started by the addition of 100 µM PLPCOOH as indicated by the arrow. The reaction was carried out in the presence or absence of GSH S-transferase pi (πGST). B. Prdx6 fluorescence emission spectrum at varying times following addition of PLPCOOH (excitation at 295 nm). Prdx6 that had been reduced by GSH-loaded πGST was incubated with PLPCOOH (100µM); πGST was removed before incubation by GST-trap chromatography. The number associated with each curve indicates the time (mins) following addition of substrate. C. Detection of Prdx6 oxidation state for the experiments described in B. Samples at each time point were analyzed by SDS-PAGE followed by Western blot using a pAb against oxidized Prdx6 (upper panel) or against total Prdx6 (lower panel). The antibody against the oxidized protein detects both the sulfinic (−SO2) and sulfonic (−SO3) states of the protein. All figures are representative results for n=3 separate experiments.