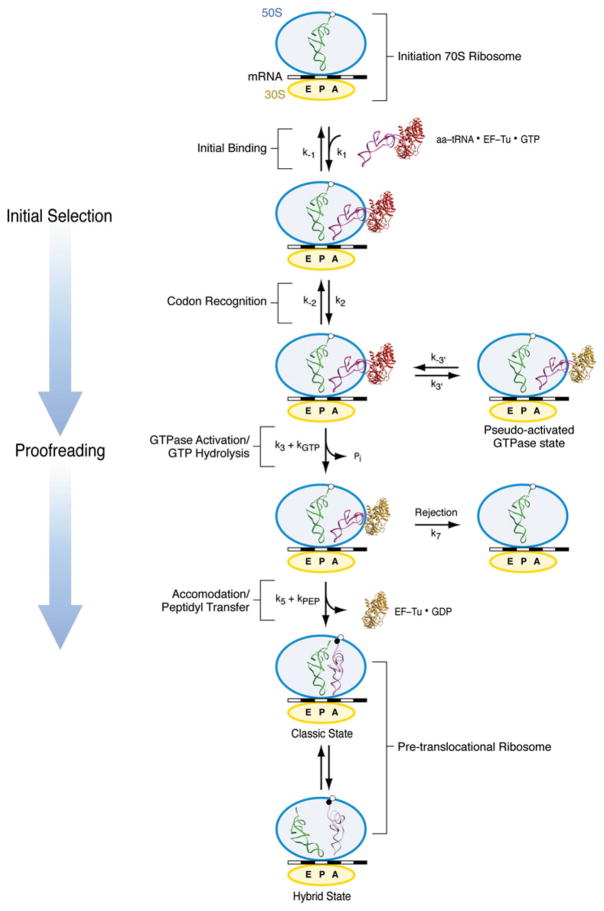
Fig. 3.
Kinetic scheme for aa-tRNA incorporation. Initial selection starts with a codon-independent, reversible rapid initial binding of the ternary complex (k1, k−1). During codon recognition, the codon–anticodon interaction is still labile and reversible (k2, k−2). Once the cognate codon–anticodon match is recognized, the ternary complex is stabilized and a signal transmitted to GTPase EF-Tu calling for activation (k3) and GTP hydrolysis (k4=kGTP). In the reversible, short life-time, pseudo-activated GTPase state, the cognate ternary complex establishes initial unsuccessful contacts with the ribosome (k3′, k−3′). Once GTP is hydrolyzed, the release of Pi and subsequent conformational change of EF-Tu leads to the dissociation of the factor from the ribosome and the accommodation of the aminoacyl end of the tRNA into the A site of the 50S subunit (k5). Accommodation is followed by rapid peptide bond transfer (k6=kpep). In case of rejection, the aa-tRNA dissociates from the ribosome (k7). After peptide bond transfer, the pre-translocational ribosome alternates between the classic and hybrid states.